Cytomegalovirus Coinfection Is Associated with Increased Vascular-Homing CD57+ CD4 T Cells in HIV Infection
- PMID: 32229536
- PMCID: PMC7315224
- DOI: 10.4049/jimmunol.1900734
Cytomegalovirus Coinfection Is Associated with Increased Vascular-Homing CD57+ CD4 T Cells in HIV Infection
Abstract
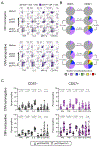
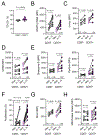
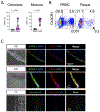
Cytotoxic CD4 T cells are linked to cardiovascular morbidities and accumulate in both HIV and CMV infections, both of which are associated with increased risk of cardiovascular disease (CVD). In this study, we identify CMV coinfection as a major driver of the cytotoxic phenotype, characterized by elevated CD57 expression and reduced CD28 expression, in circulating CD4 T cells from people living with HIV infection, and investigate potential mechanisms linking this cell population to CVD. We find that human CD57+ CD4 T cells express high levels of the costimulatory receptor CD2 and that CD2/LFA-3 costimulation results in a more robust and polyfunctional effector response to TCR signals, compared with CD28-mediated costimulation. CD57+ CD4 T cells also express the vascular endothelium-homing receptor CX3CR1 and migrate toward CX3CL1-expressing endothelial cells in vitro. IL-15 promotes the cytotoxic phenotype, elevates CX3CR1 expression, and enhances the trafficking of CD57+ CD4 T cells to endothelium and may therefore be important in linking these cells to cardiovascular complications. Finally, we demonstrate the presence of activated CD57+ CD4 T cells and expression of CX3CL1 and LFA-3 in atherosclerotic plaque tissues from HIV-uninfected donors. Our findings are consistent with a model in which cytotoxic CD4 T cells contribute to CVD in HIV/CMV coinfection and in atherosclerosis via CX3CR1-mediated trafficking and CD2/LFA-3-mediated costimulation. This study identifies several targets for therapeutic interventions and may help bridge the gap in understanding how CMV infection and immunity are linked to increased cardiovascular risk in people living with HIV infection.
Copyright © 2020 by The American Association of Immunologists, Inc.
Conflict of interest statement
CONFLICTS OF INTEREST
N.T.F serves as a consultant for Gilead. The work of L.M. was funded by the NICHD Intramural Program. All other authors declare no competing interests.
Figures

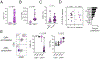

Similar articles
-
Novel Strategies to Combat CMV-Related Cardiovascular Disease.Pathog Immun. 2020 Sep 20;5(1):240-274. doi: 10.20411/pai.v5i1.382. eCollection 2020. Pathog Immun. 2020. PMID: 33089035 Free PMC article. Review.
-
Cytomegalovirus Infection Facilitates the Costimulation of CD57+CD28- CD8 T Cells in HIV Infection and Atherosclerosis via the CD2-LFA-3 Axis.J Immunol. 2024 Jan 15;212(2):245-257. doi: 10.4049/jimmunol.2300267. J Immunol. 2024. PMID: 38047900 Free PMC article.
-
A role for cytomegalovirus-specific CD4+CX3CR1+ T cells and cytomegalovirus-induced T-cell immunopathology in HIV-associated atherosclerosis.AIDS. 2012 Apr 24;26(7):805-14. doi: 10.1097/QAD.0b013e328351f780. AIDS. 2012. PMID: 22313962 Free PMC article.
-
Impact of HIV on CD8+ T cell CD57 expression is distinct from that of CMV and aging.PLoS One. 2014 Feb 27;9(2):e89444. doi: 10.1371/journal.pone.0089444. eCollection 2014. PLoS One. 2014. PMID: 24586783 Free PMC article.
-
The spreading of HIV-1 infection in the human organism is caused by fractalkine trafficking of the infected lymphocytes--a review, hypothesis and implications for treatment.Virus Genes. 2007 Apr;34(2):93-109. doi: 10.1007/s11262-006-0056-x. Virus Genes. 2007. PMID: 17151939 Review.
Cited by
-
Novel Strategies to Combat CMV-Related Cardiovascular Disease.Pathog Immun. 2020 Sep 20;5(1):240-274. doi: 10.20411/pai.v5i1.382. eCollection 2020. Pathog Immun. 2020. PMID: 33089035 Free PMC article. Review.
-
Inflammation in HIV and Its Impact on Atherosclerotic Cardiovascular Disease.Circ Res. 2024 May 24;134(11):1515-1545. doi: 10.1161/CIRCRESAHA.124.323891. Epub 2024 May 23. Circ Res. 2024. PMID: 38781301 Free PMC article. Review.
-
Characterizing Epstein-Barr virus infection of the central nervous system in Zambian adults living with HIV.J Neurovirol. 2023 Dec;29(6):706-712. doi: 10.1007/s13365-023-01178-4. Epub 2023 Oct 30. J Neurovirol. 2023. PMID: 37902948
-
Inflammatory and Immune Mechanisms for Atherosclerotic Cardiovascular Disease in HIV.Int J Mol Sci. 2024 Jul 1;25(13):7266. doi: 10.3390/ijms25137266. Int J Mol Sci. 2024. PMID: 39000373 Free PMC article. Review.
-
CD4+ T cells expressing CX3CR1, GPR56, with variable CD57 are associated with cardiometabolic diseases in persons with HIV.Front Immunol. 2023 Feb 14;14:1099356. doi: 10.3389/fimmu.2023.1099356. eCollection 2023. Front Immunol. 2023. PMID: 36865544 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

