The journey to a respiratory syncytial virus vaccine
- PMID: 32217187
- PMCID: PMC7311299
- DOI: 10.1016/j.anai.2020.03.017
The journey to a respiratory syncytial virus vaccine
Abstract
Objective: The high burden associated with respiratory syncytial virus (RSV) has made the development of RSV vaccine(s) a global health high priority. This review summarizes the journey to an RSV vaccine, the different strategies and challenges associated with the development of preventive strategies for RSV, and the diverse products that are undergoing clinical testing.
Data sources: Studies on RSV biology, immunology, epidemiology, and monoclonal antibodies (mAbs) and vaccines were searched using MEDLINE. We also searched PATH.org and ClinicalTrials.gov for updated information regarding the status of RSV vaccines and mAbs undergoing clinical trials.
Study selections: We selected relevant studies conducted in infants and young children, pregnant women, and elderly population for the prevention of RSV infection.
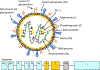
Results: Identification of a safe and immunogenic vaccine has been an important but elusive initiative for more than 60 years for different reasons, including the legacy of formalin-inactivated vaccine, our limited understanding of the immune response to RSV and how it relates to clinical disease severity, or the need for different end points according to the different vaccine platforms. Nevertheless, there are currently 39 vaccines and mAbs under development and 19 undergoing clinical trials.
Conclusion: Over the past decade, there have been significant advances in our knowledge of RSV molecular and structural biology and in understanding the human immune response to RSV. Despite the barriers, there are several promising mAbs and RSV vaccines undergoing clinical trials that hope to offer protection to the most vulnerable populations.
Copyright © 2020 American College of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of Interest
AM has received fees for participation in advisory boards from Janssen and Roche, and fees for lectures from Abbvie. RR has received fees for participation in advisory boards and lectures from Abbvie. MEP has received fees for participation in an advisory board from ReViral and lectures from Pfizer. OR has received fees for participation in advisory boards from Merck, MedImmune/Sanofi-Pasteur, and Pfizer; and fees for lectures from Pfizer.
Figures
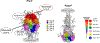

Similar articles
-
Respiratory syncytial virus vaccine: where are we now and what comes next?Expert Opin Biol Ther. 2018 Dec;18(12):1247-1256. doi: 10.1080/14712598.2018.1544239. Epub 2018 Nov 14. Expert Opin Biol Ther. 2018. PMID: 30426788 Review.
-
Summary of the Vaccines and Related Biological Products Advisory Committee meeting held to consider evaluation of vaccine candidates for the prevention of respiratory syncytial virus disease in RSV-naïve infants.Vaccine. 2020 Jan 10;38(2):101-106. doi: 10.1016/j.vaccine.2019.10.048. Epub 2019 Nov 6. Vaccine. 2020. PMID: 31706809
-
A Single-Dose Recombinant Parainfluenza Virus 5-Vectored Vaccine Expressing Respiratory Syncytial Virus (RSV) F or G Protein Protected Cotton Rats and African Green Monkeys from RSV Challenge.J Virol. 2017 May 12;91(11):e00066-17. doi: 10.1128/JVI.00066-17. Print 2017 Jun 1. J Virol. 2017. PMID: 28298602 Free PMC article.
-
Vaccines against human respiratory syncytial virus in clinical trials, where are we now?Expert Rev Vaccines. 2019 Oct;18(10):1053-1067. doi: 10.1080/14760584.2019.1675520. Epub 2019 Oct 14. Expert Rev Vaccines. 2019. PMID: 31587585 Review.
-
Nucleic acid-based vaccines targeting respiratory syncytial virus: Delivering the goods.Hum Vaccin Immunother. 2017 Nov 2;13(11):2626-2629. doi: 10.1080/21645515.2017.1363134. Hum Vaccin Immunother. 2017. PMID: 28881156 Free PMC article.
Cited by
-
Whole-Genome Sequencing and Genetic Diversity of Human Respiratory Syncytial Virus in Patients with Influenza-like Illness in Sicily (Italy) from 2017 to 2023.Viruses. 2024 May 26;16(6):851. doi: 10.3390/v16060851. Viruses. 2024. PMID: 38932144 Free PMC article.
-
Primary prevention: Vaccines and the allergist-immunologist's role.Ann Allergy Asthma Immunol. 2020 Jul;125(1):1. doi: 10.1016/j.anai.2020.03.031. Ann Allergy Asthma Immunol. 2020. PMID: 32564925 Free PMC article. No abstract available.
-
Vaccines for Respiratory Viruses-COVID and Beyond.Vaccines (Basel). 2024 Aug 22;12(8):936. doi: 10.3390/vaccines12080936. Vaccines (Basel). 2024. PMID: 39204059 Free PMC article. Review.
-
Respiratory Syncytial Virus Prevention through Monoclonal Antibodies: A Cross-Sectional Study on Knowledge, Attitudes, and Practices of Italian Pediatricians.Pediatr Rep. 2023 Feb 20;15(1):154-174. doi: 10.3390/pediatric15010013. Pediatr Rep. 2023. PMID: 36810343 Free PMC article.
-
RSV Prevention in All Infants: Which Is the Most Preferable Strategy?Front Immunol. 2022 Apr 28;13:880368. doi: 10.3389/fimmu.2022.880368. eCollection 2022. Front Immunol. 2022. PMID: 35572550 Free PMC article. Review.
References
-
- Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980–1996. Jama. 1999;282:1440–1446. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

