Suppression of a Subset of Interferon-Induced Genes by Human Papillomavirus Type 16 E7 via a Cyclin Dependent Kinase 8-Dependent Mechanism
- PMID: 32183180
- PMCID: PMC7150855
- DOI: 10.3390/v12030311
Suppression of a Subset of Interferon-Induced Genes by Human Papillomavirus Type 16 E7 via a Cyclin Dependent Kinase 8-Dependent Mechanism
Abstract
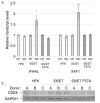
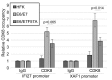
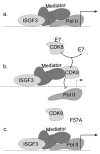
Persistent infection by human papillomaviruses (HPVs), small, double-stranded DNA viruses that infect keratinocytes of the squamous epithelia, can lead to the development of cervical and other cancers. The viral oncoprotein E7 contributes to viral persistence in part by regulating host gene expression through binding host transcriptional regulators, although mechanisms responsible for E7-mediated transcriptional regulation are incompletely understood. Type I IFN signaling promotes the expression of anti-viral genes, called interferon-stimulated genes (ISGs), through the phosphorylation and activation of STAT1. In this study, we have observed that the CR3 domain of E7 contributes to the episomal maintenance of viral genomes. Transcriptome analysis revealed that E7 transcriptionally suppresses a subset of ISGs but not through regulation of STAT1 activation. Instead, we discovered that E7 associates with Mediator kinase CDK8 and this is correlated with the recruitment of CDK8 to ISG promoters and reduced ISG expression. E7 fails to suppress ISGs in the absence of CDK8, indicating that CDK8 function contributes to the suppression of ISGs by E7. Altogether, E7/CDK8 association may be a novel mechanism by which E7 inhibits innate immune signaling.
Keywords: IFN signaling; Mediator kinase CDK8; STAT1; interferon-stimulated genes; oncoprotein E7; papillomaviruses; transcription.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
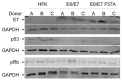
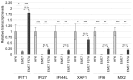
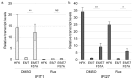

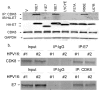
Similar articles
-
The Human Papillomavirus 16 E7 Oncoprotein Attenuates AKT Signaling To Promote Internal Ribosome Entry Site-Dependent Translation and Expression of c-MYC.J Virol. 2016 May 27;90(12):5611-5621. doi: 10.1128/JVI.00411-16. Print 2016 Jun 15. J Virol. 2016. PMID: 27030265 Free PMC article.
-
Human Papillomavirus E7 Oncoprotein Subverts Host Innate Immunity via SUV39H1-Mediated Epigenetic Silencing of Immune Sensor Genes.J Virol. 2020 Jan 31;94(4):e01812-19. doi: 10.1128/JVI.01812-19. Print 2020 Jan 31. J Virol. 2020. PMID: 31776268 Free PMC article.
-
Human Papillomavirus 16 E5 Inhibits Interferon Signaling and Supports Episomal Viral Maintenance.J Virol. 2020 Jan 6;94(2):e01582-19. doi: 10.1128/JVI.01582-19. Print 2020 Jan 6. J Virol. 2020. PMID: 31666385 Free PMC article.
-
The papillomavirus E7 proteins.Virology. 2013 Oct;445(1-2):138-68. doi: 10.1016/j.virol.2013.04.013. Epub 2013 May 31. Virology. 2013. PMID: 23731972 Free PMC article. Review.
-
The human papillomavirus E7 oncoprotein as a regulator of transcription.Virus Res. 2017 Mar 2;231:56-75. doi: 10.1016/j.virusres.2016.10.017. Epub 2016 Nov 8. Virus Res. 2017. PMID: 27818212 Free PMC article. Review.
Cited by
-
Unraveling Immunological Dynamics: HPV Infection in Women-Insights from Pregnancy.Viruses. 2023 Sep 27;15(10):2011. doi: 10.3390/v15102011. Viruses. 2023. PMID: 37896788 Free PMC article. Review.
-
Exploitation of the Mediator complex by viruses.PLoS Pathog. 2022 Apr 21;18(4):e1010422. doi: 10.1371/journal.ppat.1010422. eCollection 2022 Apr. PLoS Pathog. 2022. PMID: 35446926 Free PMC article. No abstract available.
-
Human papillomavirus associated cervical lesion: pathogenesis and therapeutic interventions.MedComm (2020). 2023 Sep 14;4(5):e368. doi: 10.1002/mco2.368. eCollection 2023 Oct. MedComm (2020). 2023. PMID: 37719443 Free PMC article. Review.
-
Inhibition of Cyclin-Dependent Kinases 8/19 Restricts Bacterial and Virus-Induced Inflammatory Responses in Monocytes.Viruses. 2023 May 31;15(6):1292. doi: 10.3390/v15061292. Viruses. 2023. PMID: 37376593 Free PMC article.
-
A luminescence-based method to assess antigen presentation and antigen-specific T cell responses for in vitro screening of immunomodulatory checkpoints and therapeutics.Front Immunol. 2023 Jul 25;14:1233113. doi: 10.3389/fimmu.2023.1233113. eCollection 2023. Front Immunol. 2023. PMID: 37559730 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

