Maternal Broadly Neutralizing Antibodies Can Select for Neutralization-Resistant, Infant-Transmitted/Founder HIV Variants
- PMID: 32156815
- PMCID: PMC7064758
- DOI: 10.1128/mBio.00176-20
Maternal Broadly Neutralizing Antibodies Can Select for Neutralization-Resistant, Infant-Transmitted/Founder HIV Variants
Abstract
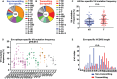
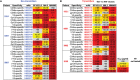
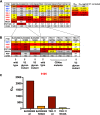
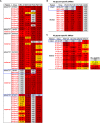
Each year, >180,000 infants become infected via mother-to-child transmission (MTCT) of HIV despite the availability of effective maternal antiretroviral treatments, underlining the need for a maternal HIV vaccine. We characterized 224 maternal HIV envelope (Env)-specific IgG monoclonal antibodies (MAbs) from seven nontransmitting and transmitting HIV-infected U.S. and Malawian mothers and examined their neutralization activities against nontransmitted autologous circulating viruses and infant-transmitted founder (infant-T/F) viruses. Only a small subset of maternal viruses, 3 of 72 (4%), were weakly neutralized by maternal linear V3 epitope-specific IgG MAbs, whereas 6 out of 6 (100%) infant-T/F viruses were neutralization resistant to these V3-specific IgG MAbs. We also show that maternal-plasma broadly neutralizing antibody (bNAb) responses targeting the V3 glycan supersite in a transmitting woman may have selected for an N332 V3 glycan neutralization-resistant infant-T/F virus. These data have important implications for bNAb-eliciting vaccines and passively administered bNAbs in the setting of MTCT.IMPORTANCE Efforts to eliminate MTCT of HIV with antiretroviral therapy (ART) have met little success, with >180,000 infant infections each year worldwide. It is therefore likely that additional immunologic strategies that can synergize with ART will be required to eliminate MTCT of HIV. To this end, understanding the role of maternal HIV Env-specific IgG antibodies in the setting of MTCT is crucial. In this study, we found that maternal-plasma broadly neutralizing antibody (bNAb) responses can select for T/F viruses that initiate infection in infants. We propose that clinical trials testing the efficacy of single bNAb specificities should not include HIV-infected pregnant women, as a single bNAb might select for neutralization-resistant infant-T/F viruses.
Keywords: HIV; MTCT; antibody; bNAbs; infant-T/F virus; mother-to-child transmission; neutralization; vertical transmission; virus.
Copyright © 2020 Martinez et al.
Figures
Similar articles
-
Vertical HIV-1 Transmission in the Setting of Maternal Broad and Potent Antibody Responses.J Virol. 2022 Jun 8;96(11):e0023122. doi: 10.1128/jvi.00231-22. Epub 2022 May 10. J Virol. 2022. PMID: 35536018 Free PMC article.
-
Mutations that confer resistance to broadly-neutralizing antibodies define HIV-1 variants of transmitting mothers from that of non-transmitting mothers.PLoS Pathog. 2021 Apr 2;17(4):e1009478. doi: 10.1371/journal.ppat.1009478. eCollection 2021 Apr. PLoS Pathog. 2021. PMID: 33798244 Free PMC article.
-
Maternal Binding and Neutralizing IgG Responses Targeting the C-Terminal Region of the V3 Loop Are Predictive of Reduced Peripartum HIV-1 Transmission Risk.J Virol. 2017 Apr 13;91(9):e02422-16. doi: 10.1128/JVI.02422-16. Print 2017 May 1. J Virol. 2017. PMID: 28202762 Free PMC article.
-
Antibodies for prevention of mother-to-child transmission of HIV-1.Curr Opin HIV AIDS. 2015 May;10(3):177-82. doi: 10.1097/COH.0000000000000150. Curr Opin HIV AIDS. 2015. PMID: 25700205 Free PMC article. Review.
-
The Role of Maternal HIV Envelope-Specific Antibodies and Mother-to-Child Transmission Risk.Front Immunol. 2017 Sep 4;8:1091. doi: 10.3389/fimmu.2017.01091. eCollection 2017. Front Immunol. 2017. PMID: 28928750 Free PMC article. Review.
Cited by
-
Leveraging antigenic seniority for maternal vaccination to prevent mother-to-child transmission of HIV-1.NPJ Vaccines. 2022 Jul 30;7(1):87. doi: 10.1038/s41541-022-00505-w. NPJ Vaccines. 2022. PMID: 35907918 Free PMC article.
-
Antibody-dependent cellular cytotoxicity responses and susceptibility influence HIV-1 mother-to-child transmission.JCI Insight. 2022 May 9;7(9):e159435. doi: 10.1172/jci.insight.159435. JCI Insight. 2022. PMID: 35324477 Free PMC article.
-
Antimalarial antibody repertoire defined by plasma IG proteomics and single B cell IG sequencing.JCI Insight. 2020 Nov 19;5(22):e143471. doi: 10.1172/jci.insight.143471. JCI Insight. 2020. PMID: 33048842 Free PMC article.
-
Characterizing HIV drug resistance in cases of vertical transmission in the VESTED randomized antiretroviral treatment trial.J Acquir Immune Defic Syndr. 2024 Aug;96(4):385-392. doi: 10.1097/qai.0000000000003435. J Acquir Immune Defic Syndr. 2024. PMID: 39175843 Clinical Trial.
-
Diversity and Function of Maternal HIV-1-Specific Antibodies at the Time of Vertical Transmission.J Virol. 2020 Apr 16;94(9):e01594-19. doi: 10.1128/JVI.01594-19. Print 2020 Apr 16. J Virol. 2020. PMID: 32075936 Free PMC article.
References
-
- UNAIDS. 2018. Global HIV & AIDS statistics—2018 fact sheet. UNAIDS, Geneva, Switzerland.
-
- Fowler MG, Fiscus SA, Currier JS, Makanani B, Martinson F, Chipato T, Browning R, Shapiro D, Mofenson L. 2015. PROMISE: efficacy and safety of 2 strategies to prevent perinatal HIV transmission. Oral presentation. Conference on Retroviruses and Opportunistic Infections, Seattle, WA.
-
- UNAIDS. 2015. Progress report on the global plan: towards the elimination of new HIV infections among children and keeping their mothers alive. UNAIDS, Geneva, Switzerland.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
