The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application
- PMID: 32150748
- PMCID: PMC7081172
- DOI: 10.7326/M20-0504
The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application
Abstract
Background: A novel human coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was identified in China in December 2019. There is limited support for many of its key epidemiologic features, including the incubation period for clinical disease (coronavirus disease 2019 [COVID-19]), which has important implications for surveillance and control activities.
Objective: To estimate the length of the incubation period of COVID-19 and describe its public health implications.
Design: Pooled analysis of confirmed COVID-19 cases reported between 4 January 2020 and 24 February 2020.
Setting: News reports and press releases from 50 provinces, regions, and countries outside Wuhan, Hubei province, China.
Participants: Persons with confirmed SARS-CoV-2 infection outside Hubei province, China.
Measurements: Patient demographic characteristics and dates and times of possible exposure, symptom onset, fever onset, and hospitalization.
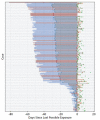
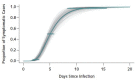
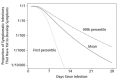
Results: There were 181 confirmed cases with identifiable exposure and symptom onset windows to estimate the incubation period of COVID-19. The median incubation period was estimated to be 5.1 days (95% CI, 4.5 to 5.8 days), and 97.5% of those who develop symptoms will do so within 11.5 days (CI, 8.2 to 15.6 days) of infection. These estimates imply that, under conservative assumptions, 101 out of every 10 000 cases (99th percentile, 482) will develop symptoms after 14 days of active monitoring or quarantine.
Limitation: Publicly reported cases may overrepresent severe cases, the incubation period for which may differ from that of mild cases.
Conclusion: This work provides additional evidence for a median incubation period for COVID-19 of approximately 5 days, similar to SARS. Our results support current proposals for the length of quarantine or active monitoring of persons potentially exposed to SARS-CoV-2, although longer monitoring periods might be justified in extreme cases.
Primary funding source: U.S. Centers for Disease Control and Prevention, National Institute of Allergy and Infectious Diseases, National Institute of General Medical Sciences, and Alexander von Humboldt Foundation.
Figures

Comment in
-
COVID-19 Follow up Testing.J Infect. 2020 Oct;81(4):647-679. doi: 10.1016/j.jinf.2020.05.012. Epub 2020 May 11. J Infect. 2020. PMID: 32407758 Free PMC article.
Similar articles
-
Evolving epidemiology and transmission dynamics of coronavirus disease 2019 outside Hubei province, China: a descriptive and modelling study.Lancet Infect Dis. 2020 Jul;20(7):793-802. doi: 10.1016/S1473-3099(20)30230-9. Epub 2020 Apr 2. Lancet Infect Dis. 2020. PMID: 32247326 Free PMC article.
-
Epidemiological Characteristics and Incubation Period of 7015 Confirmed Cases With Coronavirus Disease 2019 Outside Hubei Province in China.J Infect Dis. 2020 Jun 16;222(1):26-33. doi: 10.1093/infdis/jiaa211. J Infect Dis. 2020. PMID: 32339231 Free PMC article.
-
Epidemiological parameters of COVID-19 and its implication for infectivity among patients in China, 1 January to 11 February 2020.Euro Surveill. 2020 Oct;25(40):2000250. doi: 10.2807/1560-7917.ES.2020.25.40.2000250. Euro Surveill. 2020. PMID: 33034281 Free PMC article.
-
Coronavirus Disease 2019 (COVID-19) and pregnancy: what obstetricians need to know.Am J Obstet Gynecol. 2020 May;222(5):415-426. doi: 10.1016/j.ajog.2020.02.017. Epub 2020 Feb 24. Am J Obstet Gynecol. 2020. PMID: 32105680 Free PMC article. Review.
-
[An update on the epidemiological characteristics of novel coronavirus pneumonia (COVID-19)].Zhonghua Liu Xing Bing Xue Za Zhi. 2020 Feb 10;41(2):139-144. doi: 10.3760/cma.j.issn.0254-6450.2020.02.002. Zhonghua Liu Xing Bing Xue Za Zhi. 2020. PMID: 32057211 Review. Chinese.
Cited by
-
Effectiveness of hydrogen peroxide as auxiliary treatment for hospitalized COVID-19 patients in Brazil: preliminary results of a randomized double-blind clinical trial.Epidemiol Health. 2021;43:e2021032. doi: 10.4178/epih.e2021032. Epub 2021 May 1. Epidemiol Health. 2021. PMID: 33957025 Free PMC article. Clinical Trial.
-
Interacting regional policies in containing a disease.Proc Natl Acad Sci U S A. 2021 May 11;118(19):e2021520118. doi: 10.1073/pnas.2021520118. Proc Natl Acad Sci U S A. 2021. PMID: 33906950 Free PMC article.
-
Computational Model on COVID-19 Pandemic Using Probabilistic Cellular Automata.SN Comput Sci. 2021;2(3):230. doi: 10.1007/s42979-021-00619-3. Epub 2021 Apr 22. SN Comput Sci. 2021. PMID: 33907736 Free PMC article.
-
Learnings from the Australian first few X household transmission project for COVID-19.Lancet Reg Health West Pac. 2022 Nov;28:100573. doi: 10.1016/j.lanwpc.2022.100573. Epub 2022 Sep 5. Lancet Reg Health West Pac. 2022. PMID: 36089928 Free PMC article.
-
Contact tracing evaluation for COVID-19 transmission in the different movement levels of a rural college town in the USA.Sci Rep. 2021 Mar 1;11(1):4891. doi: 10.1038/s41598-021-83722-y. Sci Rep. 2021. PMID: 33649364 Free PMC article.
References
-
- World Health Organization. Coronavirus disease 2019 (COVID-19): Situation Report – 38. 27 February 2020. Accessed at www.who.int/docs/default-source/coronaviruse/situation-reports/20200227-.... on 28 February 2020.
-
- World Health Organization. Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV). 30 January 2020. Accessed at https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-... on 31 January 2020.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
