Targeting immunometabolism as an anti-inflammatory strategy
- PMID: 32132672
- PMCID: PMC7118080
- DOI: 10.1038/s41422-020-0291-z
Targeting immunometabolism as an anti-inflammatory strategy
Abstract
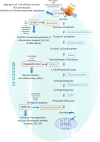
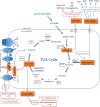
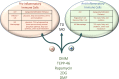
The growing field of immunometabolism has taught us how metabolic cellular reactions and processes not only provide a means to generate ATP and biosynthetic precursors, but are also a way of controlling immunity and inflammation. Metabolic reprogramming of immune cells is essential for both inflammatory as well as anti-inflammatory responses. Four anti-inflammatory therapies, DMF, Metformin, Methotrexate and Rapamycin all work by affecting metabolism and/or regulating or mimicking endogenous metabolites with anti-inflammatory effects. Evidence is emerging for the targeting of specific metabolic events as a strategy to limit inflammation in different contexts. Here we discuss these recent developments and speculate on the prospect of targeting immunometabolism in the effort to develop novel anti-inflammatory therapeutics. As accumulating evidence for roles of an intricate and elaborate network of metabolic processes, including lipid, amino acid and nucleotide metabolism provides key focal points for developing new therapies, we here turn our attention to glycolysis and the TCA cycle to provide examples of how metabolic intermediates and enzymes can provide potential novel therapeutic targets.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Dimethyl fumarate targets GAPDH and aerobic glycolysis to modulate immunity.Science. 2018 Apr 27;360(6387):449-453. doi: 10.1126/science.aan4665. Epub 2018 Mar 29. Science. 2018. PMID: 29599194 Free PMC article.
-
Krebs cycle derivatives, dimethyl fumarate and itaconate, control metabolic reprogramming in inflammatory human microglia cell line.Mitochondrion. 2024 Nov;79:101966. doi: 10.1016/j.mito.2024.101966. Epub 2024 Sep 12. Mitochondrion. 2024. PMID: 39276907
-
Metabolic Modulation in Macrophage Effector Function.Front Immunol. 2018 Feb 19;9:270. doi: 10.3389/fimmu.2018.00270. eCollection 2018. Front Immunol. 2018. PMID: 29520272 Free PMC article. Review.
-
Targeting immunometabolism against acute lung injury.Clin Immunol. 2023 Apr;249:109289. doi: 10.1016/j.clim.2023.109289. Epub 2023 Mar 12. Clin Immunol. 2023. PMID: 36918041 Free PMC article. Review.
-
Immunomodulation by dimethyl fumarate treatment improves mouse sciatic nerve regeneration.Brain Res Bull. 2020 Jul;160:24-32. doi: 10.1016/j.brainresbull.2020.04.005. Epub 2020 Apr 17. Brain Res Bull. 2020. PMID: 32305403
Cited by
-
NMR-based metabolomics identification of potential serum biomarkers of disease progression in patients with multiple sclerosis.Sci Rep. 2024 Jun 26;14(1):14806. doi: 10.1038/s41598-024-64490-x. Sci Rep. 2024. PMID: 38926483 Free PMC article.
-
G-Protein-Coupled Receptor 91-Dependent Signalling Does Not Influence Vascular Inflammation and Atherosclerosis in Hyperlipidaemic Mice.Cells. 2023 Nov 6;12(21):2580. doi: 10.3390/cells12212580. Cells. 2023. PMID: 37947659 Free PMC article.
-
Innate Immunity Effector Cells as Inflammatory Drivers of Cardiac Fibrosis.Int J Mol Sci. 2020 Sep 28;21(19):7165. doi: 10.3390/ijms21197165. Int J Mol Sci. 2020. PMID: 32998408 Free PMC article. Review.
-
Metabolic consequences of obesity and type 2 diabetes: Balancing genes and environment for personalized care.Cell. 2021 Mar 18;184(6):1530-1544. doi: 10.1016/j.cell.2021.02.012. Epub 2021 Mar 5. Cell. 2021. PMID: 33675692 Free PMC article. Review.
-
Wasp Venom Biochemical Components and Their Potential in Biological Applications and Nanotechnological Interventions.Toxins (Basel). 2021 Mar 12;13(3):206. doi: 10.3390/toxins13030206. Toxins (Basel). 2021. PMID: 33809401 Free PMC article. Review.
References
-
- Kelly B, et al. Metformin inhibits the production of reactive oxygen species from NADH:Ubiquinone oxidoreductase to limit induction of interleukin-1beta (IL-1beta) and boosts interleukin-10 (IL-10) in lipopolysaccharide (LPS)-activated macrophages. J. Biol. Chem. 2015;290:20348–20359. doi: 10.1074/jbc.M115.662114. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

