Engineering a Proximity-Directed O-GlcNAc Transferase for Selective Protein O-GlcNAcylation in Cells
- PMID: 32119511
- PMCID: PMC7296736
- DOI: 10.1021/acschembio.0c00074
Engineering a Proximity-Directed O-GlcNAc Transferase for Selective Protein O-GlcNAcylation in Cells
Abstract
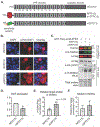
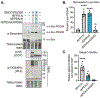
O-Linked β-N-acetylglucosamine (O-GlcNAc) is a monosaccharide that plays an essential role in cellular signaling throughout the nucleocytoplasmic proteome of eukaryotic cells. Strategies for selectively increasing O-GlcNAc levels on a target protein in cells would accelerate studies of this essential modification. Here, we report a generalizable strategy for introducing O-GlcNAc into selected target proteins in cells using a nanobody as a proximity-directing agent fused to O-GlcNAc transferase (OGT). Fusion of a nanobody that recognizes GFP (nGFP) or a nanobody that recognizes the four-amino acid sequence EPEA (nEPEA) to OGT yielded nanobody-OGT constructs that selectively delivered O-GlcNAc to a series of tagged target proteins (e.g., JunB, cJun, and Nup62). Truncation of the tetratricopeptide repeat domain as in OGT(4) increased selectivity for the target protein through the nanobody by reducing global elevation of O-GlcNAc levels in the cell. Quantitative chemical proteomics confirmed the increase in O-GlcNAc to the target protein by nanobody-OGT(4). Glycoproteomics revealed that nanobody-OGT(4) or full-length OGT produced a similar glycosite profile on the target protein JunB and Nup62. Finally, we demonstrate the ability to selectively target endogenous α-synuclein for O-GlcNAcylation in HEK293T cells. These first proximity-directed OGT constructs provide a flexible strategy for targeting additional proteins and a template for further engineering of OGT and the O-GlcNAc proteome in the future. The use of a nanobody to redirect OGT substrate selection for glycosylation of desired proteins in cells may further constitute a generalizable strategy for controlling a broader array of post-translational modifications in cells.
Figures



Similar articles
-
O-GlcNAc Engineering on a Target Protein in Cells with Nanobody-OGT and Nanobody-splitOGA.Curr Protoc. 2021 May;1(5):e117. doi: 10.1002/cpz1.117. Curr Protoc. 2021. PMID: 33950562 Free PMC article.
-
Truncation of the TPR domain of OGT alters substrate and glycosite selection.Anal Bioanal Chem. 2021 Dec;413(30):7385-7399. doi: 10.1007/s00216-021-03731-8. Epub 2021 Nov 2. Anal Bioanal Chem. 2021. PMID: 34725712 Free PMC article.
-
O-GlcNAcylation of Thr12/Ser56 in short-form O-GlcNAc transferase (sOGT) regulates its substrate selectivity.J Biol Chem. 2019 Nov 8;294(45):16620-16633. doi: 10.1074/jbc.RA119.009085. Epub 2019 Sep 16. J Biol Chem. 2019. PMID: 31527085 Free PMC article.
-
O-GlcNAc transferase congenital disorder of glycosylation (OGT-CDG): Potential mechanistic targets revealed by evaluating the OGT interactome.J Biol Chem. 2024 Sep;300(9):107599. doi: 10.1016/j.jbc.2024.107599. Epub 2024 Jul 24. J Biol Chem. 2024. PMID: 39059494 Free PMC article. Review.
-
Nucleocytoplasmic O-glycosylation: O-GlcNAc and functional proteomics.Biochimie. 2001 Jul;83(7):575-81. doi: 10.1016/s0300-9084(01)01295-0. Biochimie. 2001. PMID: 11522385 Review.
Cited by
-
O-GlcNAc informatics: advances and trends.Anal Bioanal Chem. 2024 Sep 18. doi: 10.1007/s00216-024-05531-2. Online ahead of print. Anal Bioanal Chem. 2024. PMID: 39294469 Review.
-
Organization of a functional glycolytic metabolon on mitochondria for metabolic efficiency.Nat Metab. 2024 Sep;6(9):1712-1735. doi: 10.1038/s42255-024-01121-9. Epub 2024 Sep 11. Nat Metab. 2024. PMID: 39261628
-
A phosphorylation-controlled switch confers cell cycle-dependent protein relocalization.Nat Cell Biol. 2024 Oct;26(10):1804-1816. doi: 10.1038/s41556-024-01495-8. Epub 2024 Aug 29. Nat Cell Biol. 2024. PMID: 39209962
-
O-GlcNAcylation in tumorigenesis and its implications for cancer therapy.J Biol Chem. 2024 Sep;300(9):107709. doi: 10.1016/j.jbc.2024.107709. Epub 2024 Aug 22. J Biol Chem. 2024. PMID: 39178944 Free PMC article. Review.
-
A phosphorylation-controlled switch confers cell cycle-dependent protein relocalization.bioRxiv [Preprint]. 2024 Jun 6:2024.06.05.597552. doi: 10.1101/2024.06.05.597552. bioRxiv. 2024. Update in: Nat Cell Biol. 2024 Oct;26(10):1804-1816. doi: 10.1038/s41556-024-01495-8. PMID: 38895347 Free PMC article. Updated. Preprint.
References
-
- Yuzwa SA, Shan X, Macauley MS, Clark T, Skorobogatko Y, Vosseller K, and Vocadlo DJ (2012) Increasing O-GlcNAc slows neurodegeneration and stabilizes tau against aggregation, Nat Chem Biol 8, 393–399. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

