Tumor-targeting oncolytic virus elicits potent immunotherapeutic vaccine responses to tumor antigens
- PMID: 32117591
- PMCID: PMC7028326
- DOI: 10.1080/2162402X.2020.1726168
Tumor-targeting oncolytic virus elicits potent immunotherapeutic vaccine responses to tumor antigens
Abstract
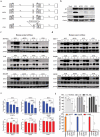
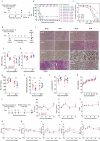
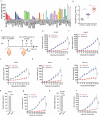
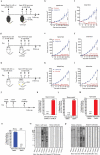
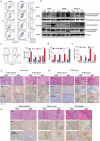
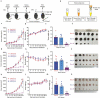
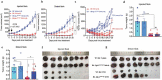
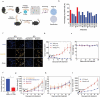
Oncolytic viruses represent a promising therapeutic modality, but they have yet to live up to their therapeutic potential. Safety and efficacy concerns impel us to identify least toxic oncolytic agents that would generate durable and multifaceted anti-tumor immune responses to disrupt the tumors. Here we describe a rational engineered oncolytic herpes virus (OVH) that is a selective killer for targeting tumors, has strong safety records, induces complete regression of tumors in multiple tumor models, and elicits potent antitumor immunity. By far, the potential of OVs in promoting the tumor antigen-specific humoral immune responses remains obscure. In this study, we found that effective treatment by OVH induced immunogenic cell death, which facilitates to elicit humoral immune responses. Depletion experiments revealed that B cells were required for maximal antitumor efficacy of oncolytic immunotherapy. Both serum transfer and antibody treatment experiments revealed that endogenous oncolysis-induced antigen-targeting therapeutic antibodies can lead to systemic tumor regression. Our data demonstrate that tumor-targeting immune modulatory properties confer oncolytic OVH virotherapy as potent immunotherapeutic cancer vaccines that can generate specific and efficacious antitumor humoral responses by eliciting endogenous tumor antigen-targeting therapeutic antibodies in situ, resulting in an efficacious and tumor-specific therapeutic effect.
Keywords: Oncolytic virus; anti-tumor effect; cancer vaccine; therapeutic antibodies; tumor-targeting.
© 2020 The Author(s). Published with license by Taylor & Francis Group, LLC.
Figures
Similar articles
-
Oncolytic virus expressing PD-1 inhibitors activates a collaborative intratumoral immune response to control tumor and synergizes with CTLA-4 or TIM-3 blockade.J Immunother Cancer. 2022 Jun;10(6):e004762. doi: 10.1136/jitc-2022-004762. J Immunother Cancer. 2022. PMID: 35688558 Free PMC article.
-
Arming oncolytic viruses to leverage antitumor immunity.Expert Opin Biol Ther. 2015 Jul;15(7):959-71. doi: 10.1517/14712598.2015.1044433. Epub 2015 May 10. Expert Opin Biol Ther. 2015. PMID: 25959450 Review.
-
Oncolytic Adenovirus and Tumor-Targeting Immune Modulatory Therapy Improve Autologous Cancer Vaccination.Cancer Res. 2017 Jul 15;77(14):3894-3907. doi: 10.1158/0008-5472.CAN-17-0468. Epub 2017 May 31. Cancer Res. 2017. PMID: 28566332 Free PMC article.
-
Oncolytic ImmunoViroTherapy: A long history of crosstalk between viruses and immune system for cancer treatment.Pharmacol Ther. 2022 Aug;236:108103. doi: 10.1016/j.pharmthera.2021.108103. Epub 2021 Dec 23. Pharmacol Ther. 2022. PMID: 34954301 Review.
-
Peptides-Coated Oncolytic Vaccines for Cancer Personalized Medicine.Front Immunol. 2022 Apr 14;13:826164. doi: 10.3389/fimmu.2022.826164. eCollection 2022. Front Immunol. 2022. PMID: 35493448 Free PMC article.
Cited by
-
Oncolytic virotherapy in cancer treatment: challenges and optimization prospects.Front Immunol. 2023 Dec 15;14:1308890. doi: 10.3389/fimmu.2023.1308890. eCollection 2023. Front Immunol. 2023. PMID: 38169820 Free PMC article. Review.
-
The potential of swine pseudorabies virus attenuated vaccine for oncolytic therapy against malignant tumors.J Exp Clin Cancer Res. 2023 Oct 27;42(1):284. doi: 10.1186/s13046-023-02848-1. J Exp Clin Cancer Res. 2023. PMID: 37891570 Free PMC article.
-
Glioblastoma Therapy: Rationale for a Mesenchymal Stem Cell-based Vehicle to Carry Recombinant Viruses.Stem Cell Rev Rep. 2022 Feb;18(2):523-543. doi: 10.1007/s12015-021-10207-w. Epub 2021 Jul 28. Stem Cell Rev Rep. 2022. PMID: 34319509 Review.
-
Mesenchymal stem cell carriers enhance anti-tumor efficacy of oncolytic virotherapy.Oncol Lett. 2021 Apr;21(4):238. doi: 10.3892/ol.2021.12499. Epub 2021 Jan 28. Oncol Lett. 2021. PMID: 33664802 Free PMC article. Review.
-
Oncolytic virus expressing PD-1 inhibitors activates a collaborative intratumoral immune response to control tumor and synergizes with CTLA-4 or TIM-3 blockade.J Immunother Cancer. 2022 Jun;10(6):e004762. doi: 10.1136/jitc-2022-004762. J Immunother Cancer. 2022. PMID: 35688558 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
