Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease
- PMID: 32114706
- PMCID: PMC7049091
- DOI: 10.1002/14651858.CD003177.pub5
Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease
Abstract
Background: Omega-3 polyunsaturated fatty acids from oily fish (long-chain omega-3 (LCn3)), including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)), as well as from plants (alpha-linolenic acid (ALA)) may benefit cardiovascular health. Guidelines recommend increasing omega-3-rich foods, and sometimes supplementation, but recent trials have not confirmed this.
Objectives: To assess the effects of increased intake of fish- and plant-based omega-3 fats for all-cause mortality, cardiovascular events, adiposity and lipids.
Search methods: We searched CENTRAL, MEDLINE and Embase to February 2019, plus ClinicalTrials.gov and World Health Organization International Clinical Trials Registry to August 2019, with no language restrictions. We handsearched systematic review references and bibliographies and contacted trial authors.
Selection criteria: We included randomised controlled trials (RCTs) that lasted at least 12 months and compared supplementation or advice to increase LCn3 or ALA intake, or both, versus usual or lower intake.
Data collection and analysis: Two review authors independently assessed trials for inclusion, extracted data and assessed validity. We performed separate random-effects meta-analysis for ALA and LCn3 interventions, and assessed dose-response relationships through meta-regression.
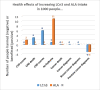
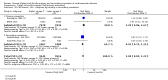
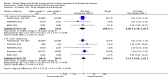
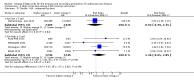
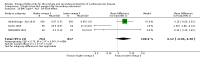
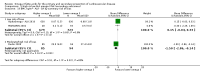
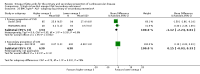
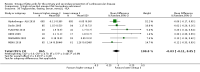
Main results: We included 86 RCTs (162,796 participants) in this review update and found that 28 were at low summary risk of bias. Trials were of 12 to 88 months' duration and included adults at varying cardiovascular risk, mainly in high-income countries. Most trials assessed LCn3 supplementation with capsules, but some used LCn3- or ALA-rich or enriched foods or dietary advice compared to placebo or usual diet. LCn3 doses ranged from 0.5 g a day to more than 5 g a day (19 RCTs gave at least 3 g LCn3 daily). Meta-analysis and sensitivity analyses suggested little or no effect of increasing LCn3 on all-cause mortality (risk ratio (RR) 0.97, 95% confidence interval (CI) 0.93 to 1.01; 143,693 participants; 11,297 deaths in 45 RCTs; high-certainty evidence), cardiovascular mortality (RR 0.92, 95% CI 0.86 to 0.99; 117,837 participants; 5658 deaths in 29 RCTs; moderate-certainty evidence), cardiovascular events (RR 0.96, 95% CI 0.92 to 1.01; 140,482 participants; 17,619 people experienced events in 43 RCTs; high-certainty evidence), stroke (RR 1.02, 95% CI 0.94 to 1.12; 138,888 participants; 2850 strokes in 31 RCTs; moderate-certainty evidence) or arrhythmia (RR 0.99, 95% CI 0.92 to 1.06; 77,990 participants; 4586 people experienced arrhythmia in 30 RCTs; low-certainty evidence). Increasing LCn3 may slightly reduce coronary heart disease mortality (number needed to treat for an additional beneficial outcome (NNTB) 334, RR 0.90, 95% CI 0.81 to 1.00; 127,378 participants; 3598 coronary heart disease deaths in 24 RCTs, low-certainty evidence) and coronary heart disease events (NNTB 167, RR 0.91, 95% CI 0.85 to 0.97; 134,116 participants; 8791 people experienced coronary heart disease events in 32 RCTs, low-certainty evidence). Overall, effects did not differ by trial duration or LCn3 dose in pre-planned subgrouping or meta-regression. There is little evidence of effects of eating fish. Increasing ALA intake probably makes little or no difference to all-cause mortality (RR 1.01, 95% CI 0.84 to 1.20; 19,327 participants; 459 deaths in 5 RCTs, moderate-certainty evidence),cardiovascular mortality (RR 0.96, 95% CI 0.74 to 1.25; 18,619 participants; 219 cardiovascular deaths in 4 RCTs; moderate-certainty evidence), coronary heart disease mortality (RR 0.95, 95% CI 0.72 to 1.26; 18,353 participants; 193 coronary heart disease deaths in 3 RCTs; moderate-certainty evidence) and coronary heart disease events (RR 1.00, 95% CI 0.82 to 1.22; 19,061 participants; 397 coronary heart disease events in 4 RCTs; low-certainty evidence). However, increased ALA may slightly reduce risk of cardiovascular disease events (NNTB 500, RR 0.95, 95% CI 0.83 to 1.07; but RR 0.91, 95% CI 0.79 to 1.04 in RCTs at low summary risk of bias; 19,327 participants; 884 cardiovascular disease events in 5 RCTs; low-certainty evidence), and probably slightly reduces risk of arrhythmia (NNTB 91, RR 0.73, 95% CI 0.55 to 0.97; 4912 participants; 173 events in 2 RCTs; moderate-certainty evidence). Effects on stroke are unclear. Increasing LCn3 and ALA had little or no effect on serious adverse events, adiposity, lipids and blood pressure, except increasing LCn3 reduced triglycerides by ˜15% in a dose-dependent way (high-certainty evidence).
Authors' conclusions: This is the most extensive systematic assessment of effects of omega-3 fats on cardiovascular health to date. Moderate- and low-certainty evidence suggests that increasing LCn3 slightly reduces risk of coronary heart disease mortality and events, and reduces serum triglycerides (evidence mainly from supplement trials). Increasing ALA slightly reduces risk of cardiovascular events and arrhythmia.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
ASA: none known TJB: none known JSB: none known PB: none known GCT: none known HJM: none known KHOD: none known FKA: none known CDS: none known HVW: none known FS: none known LH: none known
Figures







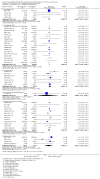





























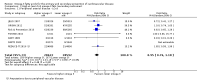
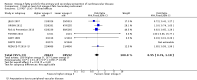


































































































































































































Update of
-
Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease.Cochrane Database Syst Rev. 2018 Nov 30;11(11):CD003177. doi: 10.1002/14651858.CD003177.pub4. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2020 Feb 29;3:CD003177. doi: 10.1002/14651858.CD003177.pub5. PMID: 30521670 Free PMC article. Updated.
Similar articles
-
Polyunsaturated fatty acids for the primary and secondary prevention of cardiovascular disease.Cochrane Database Syst Rev. 2018 Jul 18;7(7):CD012345. doi: 10.1002/14651858.CD012345.pub2. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2018 Nov 27;11:CD012345. doi: 10.1002/14651858.CD012345.pub3. PMID: 30019767 Free PMC article. Updated. Review.
-
Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease.Cochrane Database Syst Rev. 2018 Nov 30;11(11):CD003177. doi: 10.1002/14651858.CD003177.pub4. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2020 Feb 29;3:CD003177. doi: 10.1002/14651858.CD003177.pub5. PMID: 30521670 Free PMC article. Updated.
-
Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease.Cochrane Database Syst Rev. 2018 Jul 18;7(7):CD003177. doi: 10.1002/14651858.CD003177.pub3. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2018 Nov 30;11:CD003177. doi: 10.1002/14651858.CD003177.pub4. PMID: 30019766 Free PMC article. Updated. Review.
-
Polyunsaturated fatty acids for the primary and secondary prevention of cardiovascular disease.Cochrane Database Syst Rev. 2018 Nov 27;11(11):CD012345. doi: 10.1002/14651858.CD012345.pub3. Cochrane Database Syst Rev. 2018. PMID: 30484282 Free PMC article.
-
Omega-6 fats for the primary and secondary prevention of cardiovascular disease.Cochrane Database Syst Rev. 2018 Nov 29;11(11):CD011094. doi: 10.1002/14651858.CD011094.pub4. Cochrane Database Syst Rev. 2018. PMID: 30488422 Free PMC article.
Cited by
-
Significance of Omega-3 Fatty Acids in the Prophylaxis and Treatment after Spinal Cord Injury in Rodent Models.Mediators Inflamm. 2020 Jul 29;2020:3164260. doi: 10.1155/2020/3164260. eCollection 2020. Mediators Inflamm. 2020. PMID: 32801994 Free PMC article. Review.
-
Can N-3 polyunsaturated fatty acids be considered a potential adjuvant therapy for COVID-19-associated cardiovascular complications?Pharmacol Ther. 2021 Mar;219:107703. doi: 10.1016/j.pharmthera.2020.107703. Epub 2020 Oct 5. Pharmacol Ther. 2021. PMID: 33031856 Free PMC article. Review.
-
Inflammation and Nutrition: Friend or Foe?Nutrients. 2023 Feb 25;15(5):1159. doi: 10.3390/nu15051159. Nutrients. 2023. PMID: 36904164 Free PMC article. Review.
-
Polyunsaturated fatty acids for the primary and secondary prevention of cardiovascular disease.Cochrane Database Syst Rev. 2018 Jul 18;7(7):CD012345. doi: 10.1002/14651858.CD012345.pub2. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2018 Nov 27;11:CD012345. doi: 10.1002/14651858.CD012345.pub3. PMID: 30019767 Free PMC article. Updated. Review.
-
Docosahexaenoic acid supplementation represses the early immune response against murine cytomegalovirus but enhances NK cell effector function.BMC Immunol. 2022 Apr 19;23(1):17. doi: 10.1186/s12865-022-00492-6. BMC Immunol. 2022. PMID: 35439922 Free PMC article.
References
References to studies included in this review
ADCS 2010 {published data only}
AFFORD 2013 {published data only}
-
- Nigam A, Talajic M, Roy D, Nattel S, Lambert J, Nozza A, et al. Fish oil for the reduction of atrial fibrillation recurrence, inflammation, and oxidative stress. Journal of the American College of Cardiology 2014;64(14):1441‐8. - PubMed
-
- Nigam A, Talajic M, Roy D, Nattel S, Lambert J, Nozza A, et al. Multicentre trial of fish oil for the reduction of atrial fibrillation recurrence, inflammation and oxidative stress: the atrial fibrillation fish oil research study. Canadian Journal of Cardiology 2013;1:S383. - PubMed
Ahn 2016 {published data only}
AlphaOmega ‐ ALA 2010 {published and unpublished data}
-
- Geleijnse J, Giltay E, Kromhout D. Effects of n‐3 fatty acids on cognitive decline: a randomized double‐blind, placebo‐controlled trial in stable myocardial infarction patients. Alzheimer's & Dementia 2011;1:S512. - PubMed
-
- Geleijnse JM, Giltay EJ, Kromhout D. Effects of n‐3 fatty acids on cognitive decline: a randomized, double‐blind, placebo‐controlled trial in stable myocardial infarction patients. Alzheimer's & Dementia 2012;8(4):278‐87. - PubMed
-
- Geleijnse JM, Giltay EJ, Schouten EG, Goede J, Oude Griep LM, Teitsma‐Jansen AM, et al. Effect of low doses of n‐3 fatty acids on cardiovascular diseases in 4,837 post‐myocardial infarction patients: design and baseline characteristics of the Alpha Omega Trial. American Heart Journal 2010;159(4):539‐46. [DOI: ] - PubMed
AlphaOmega ‐ EPA+DHA 2010 {published and unpublished data}
-
- Aung T, Halsey J, Kromhout D, Gerstein HC, Marchioli R, Tavazzi L, et al. Associations of omega‐3 fatty acid supplement use with cardiovascular disease risks: meta‐analysis of 10 trials involving 77 917 individuals. JAMA Cardiology 2018;3(3):225‐34. [DOI: 10.1001/jamacardio.2017.5205] - DOI - PMC - PubMed
-
- Geleijnse J, Giltay E, Kromhout D. Effects of n‐3 fatty acids on cognitive decline: a randomized double‐blind, placebo‐controlled trial in stable myocardial infarction patients. Alzheimer's & Dementia 2011;1:S512. - PubMed
-
- Geleijnse JM, Giltay EJ, Kromhout D. Effects of n‐3 fatty acids on cognitive decline: a randomized, double‐blind, placebo‐controlled trial in stable myocardial infarction patients. Alzheimer's & Dementia 2012;8(4):278‐87. - PubMed
AREDS2 2014 {published and unpublished data}
-
- Age‐Related Eye Disease Study 2 (AREDS2) Research Group. Lutein + zeaxanthin and omega‐3 fatty acids for age‐related macular degeneration: the Age‐Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013;309(19):2005‐15. [PUBMED: 23644932] - PubMed
-
- Aung T, Halsey J, Kromhout D, Gerstein HC, Marchioli R, Tavazzi L, et al. Associations of omega‐3 fatty acid supplement use with cardiovascular disease risks: meta‐analysis of 10 trials involving 77 917 individuals. JAMA Cardiology 2018;3(3):225‐34. [DOI: 10.1001/jamacardio.2017.5205] - DOI - PMC - PubMed
ASCEND 2018 {published data only}
-
- Bowman L, Aung T, Haynes R, Armitage J. ASCEND: design and baseline characteristics of a large randomised trial in diabetes. Diabetes 2012;61:A556‐7.
-
- NCT00135226. ASCEND: a study of cardiovascular events in diabetes [A study of cardiovascular events in diabetes ‐ a randomized 2x2 factorial study of aspirin versus placebo, and of omega‐3 fatty acid supplementation versus placebo, for primary prevention of cardiovascular events in people with diabetes]. clinicaltrials.gov/ct2/show/NCT00135226 (first received 25 August 2005).
Baldassarre 2006 {published data only}
-
- Baldassarre D, Amato M, Eligini S, Barbieri SS, Mussoni L, Frigerio B, et al. Effect of n‐3 fatty acids on carotid atherosclerosis and haemostasis in patients with combined hyperlipoproteinemia: a double‐blind pilot study in primary prevention. Annals of Medicine 2006;38(5):367‐75. [DOI: 10.1080/07853890600852880] - DOI - PubMed
Bates 1989 {published data only}
Berson 2004 {published data only}
-
- Berson EL, Rosner B, Sandberg MA, Weigel‐DiFranco C, Moser A, Brockhurst RJ, et al. Clinical trial of docosahexaenoic acid in patients with retinitis pigmentosa receiving vitamin A treatment. Archives of Ophthalmology 2004;122(9):1297‐305. - PubMed
-
- Berson EL, Rosner B, Sandberg MA, Weigel‐DiFranco C, Moser A, Brockhurst RJ, et al. Further evaluation of docosahexaenoic acid in patients with retinitis pigmentosa receiving vitamin A treatment: subgroup analyses. Archives of Ophthalmology 2004;122(9):1306‐14. - PubMed
Broutset 2007 {published data only}
-
- Lanzmann‐Petithory D, Broustet J‐P, Flammang D, Sorain F, Combe N, LaBelle RS, et al. Prevention of atrial fibrillation recurrence with an α‐linolenic acid enriched diet: a randomized study. Journal of Clinical Lipidology 2007;1(5):524, abstract 439.
-
- NCT00410020. Arrhythmia prevention with an alpha‐linolenic enriched diet [Secondary prevention of atrial fibrillation with an alpha‐linolenic enriched diet: a randomized study]. clinicaltrials.gov/ct2/show/NCT00410020 (first received 12 December 2006).
Brox 2001 {published and unpublished data}
-
- Brox J, Olaussen K, Osterud B, Elvevoll EO, Bjornstad E, Brattebog G, et al. A long‐term seal‐ and cod‐liver‐oil supplementation in hypercholesterolemic subjects. Lipids 2001;36(1):7‐13. - PubMed
Caldwell 2011 {published data only}
-
- Caldwell SH, Argo CK, Henry TD, Lackner C, Pramoonjago P, Weltman AL, et al. Dissociated histological and metabolic effects of omega‐3 (3000 mg/d) versus placebo with both exercise and diet in a double‐blind randomized controlled trial of NASH. Journal of Hepatology 2011; Vol. 54, issue Supplement 1:S8.
DART 1989 {published and unpublished data}
-
- Burr ML, Fehily AM. Fatty fish and heart disease: a randomized controlled trial. World Review of Nutrition and Dietetics 1991;66:306‐12. - PubMed
-
- Burr ML, Fehily AM. Fish and the heart. Lancet 1989;ii:1451‐2.
-
- Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, et al. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet 1989;2(8666):757‐61. - PubMed
-
- Burr ML, Fehily AM, Rogers S, Welsby E, King S, Sandham S. Diet and reinfarction trial (DART): design, recruitment, and compliance. European Heart Journal 1989;10(6):558‐67. - PubMed
-
- Burr ML, Holliday RM, Fehily AM, Whitehead PJ. Haematological prognostic indices after myocardial infarction: evidence from the diet and reinfarction trial (DART). European Heart Journal 1992;13(2):166‐70. - PubMed
DART2 2003 {published and unpublished data}
-
- Burr ML. Secondary prevention of CHD in UK men: the diet and reinfarction trial and its sequel. Proceedings of the Nutrition Society 2007;66(1):9‐15. [PUBMED: 17343767] - PubMed
-
- Burr ML, Ashfield‐Watt PA, Dunstan FD, Fehily AM, Breay P, Ashton T, et al. Lack of benefit of dietary advice to men with angina: results of a controlled trial. European Journal of Clinical Nutrition 2003;57(2):193‐200. - PubMed
-
- Burr ML, Dunstan FD, George CH. Is fish oil good or bad for heart disease? Two trials with apparently conflicting results. Journal of Membrane Biology 2005;206(2):155‐63. [PUBMED: 16456725] - PubMed
-
- Ness AR, Ashfield‐Watt PA, Whiting JM, Smith GD, Hughes J, Burr ML. The long‐term effect of dietary advice on the diet of men with angina: the diet and angina randomized trial. Journal of Human Nutrition and Dietetics 2004;17:1‐3. - PubMed
-
- Ness AR, Gallacher JE, Bennett PD, Gunnell DJ, Rogers PJ, Kessler D, et al. Advice to eat fish and mood: a randomised controlled trial in men with angina. Nutritional Neuroscience 2003;6(1):63‐5. - PubMed
Derosa 2016 {published and unpublished data}
-
- Derosa G, Cicero AF, D'Angelo A, Borghi C, Maffioli P. Effects of n‐3 PUFAs on fasting plasma glucose and insulin resistance in patients with impaired fasting glucose or impaired glucose tolerance. BioFactors (Oxford, England) 2016;42(3):316‐22. [PUBMED: 27040503] - PubMed
Deslypere 1992 {published and unpublished data}
-
- Blok WL, Deslypere JP, Demacker PN, Ven‐Jongekrijg J, Hectors MP, Meer JW, et al. Pro‐ and anti‐inflammatory cytokines in healthy volunteers fed various doses of fish oil for 1 year. European Journal of Clinical Investigation 1997;27(12):1003‐8. [PUBMED: 9466128] - PubMed
-
- Deslypere JP. Influence of supplementation with n‐3 fatty acids on different coronary risk factors in men: a placebo controlled study. Verhandelingen ‐ Koninklijke Academie voor Geneeskunde van Belgie 1992;54(3):189‐216. [PUBMED: 1413984] - PubMed
-
- Katan MB, Deslypere JP, Birgelen AP, Penders M, Zegwaard M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18‐month controlled study. Journal of Lipid Research 1997;38(10):2012‐22. [PUBMED: 9374124] - PubMed
DIPP 2015 {published and unpublished data}
-
- Tokudome S, Kuriki K, Yokoyama Y, Sasaki M, Joh T, Kamiya T, et al. Dietary n‐3/long‐chain n‐3 polyunsaturated fatty acids for prevention of sporadic colorectal tumors: a randomized controlled trial in polypectomized participants. Prostaglandins Leukotrienes and Essential Fatty Acids 2015;94:1‐11. [PUBMED: 25451556] - PubMed
-
- Tokudome S, Yokoyama Y, Kamiya T, Seno K, Okuyama H, Kuriki K, et al. Rationale and study design of dietary intervention in patients polypectomized for tumors of the colorectum. Japanese Journal of Clinical Oncology 2002;32(12):550‐3. [PUBMED: 12578906] - PubMed
DISAF 2003 {published and unpublished data}
-
- Harrison RA. Dietary Intervention for Maintaining Sinus Rhythm Following Cardioversion for Atrial Fibrillation: a Randomised Controlled Trial [PhD thesis]. Manchester (UK): Faculty of Medicine, Dentistry, Nursing and Pharmacy, 2005.
-
- Harrison RA, Elton P. From pies to pilchards: dietary assistants increase consumption of oil rich fish. Journal of Epidemiology and Community Health 2000;Suppl:6.
-
- Harrison RA, Elton PJ. Can an oil‐rich fish diet improve treatment outcomes following cardioversion for atrial fibrillation? A randomised controlled trial. Study design and compliance. International Society for the Study of Fatty Acids and Lipids (ISSFAL); 2002 May; Montreal, Canada. 2002.
-
- Harrison RA, Elton PJ. Is there a role for long‐chain omega3 or oil‐rich fish in the treatment of atrial fibrillation?. Medical Hypotheses 2005;64(1):59‐63. [PUBMED: 15533612] - PubMed
-
- Harrison RA, Purnell P, Elton PJ. Using community‐based dietary assistants to increase the intake of oil‐rich fish among older people. European Journal of Public Health 2003;13(Suppl 1):105.
Dodin 2005 {published data only}
-
- Dodin S, Cunnane SC, Masse B, Lemay A, Jacques H, Asselin G, et al. Flaxseed on cardiovascular disease markers in healthy menopausal women: a randomized, double‐blind, placebo‐controlled trial. Nutrition (Burbank, Los Angeles County, Calif.) 2008;24(1):23‐30. [PUBMED: 17981439] - PubMed
-
- Dodin S, Lemay A, Jacques H, Legare F, Forest JC, Masse B. The effects of flaxseed dietary supplement on lipid profile, bone mineral density, and symptoms in menopausal women: a randomized, double‐blind, wheat germ placebo‐controlled clinical trial. Journal of Clinical Endocrinology and Metabolism 2005;90(3):1390‐7. [PUBMED: 15613422] - PubMed
Doi 2014 {published data only}
-
- Doi M, Nosaka K, Miyoshi T, Iwamoto M, Kajiya M, Okawa K, et al. Clinical outcomes of early initiation of pure eicosapentaenoic acid supplement after percutaneous coronary intervention in patients with acute coronary syndrome. European Heart Journal 2014;35(Abstract Suppl):1156.
-
- Doi M, Nosaka K, Miyoshi T, Iwamoto M, Kajiya M, Okawa K, et al. Early eicosapentaenoic acid treatment after percutaneous coronary intervention reduces acute inflammatory responses and ventricular arrhythmias in patients with acute myocardial infarction: a randomized, controlled study. International Journal of Cardiology 2014;176(3):577‐82. [PUBMED: 25305703] - PubMed
-
- Nosaka K, Miyoshi T, Iwamoto M, Kajiya M, Okawa K, Tsukuda S, et al. Early initiation of eicosapentaenoic acid and statin treatment is associated with better clinical outcomes than statin alone in patients with acute coronary syndromes: 1‐year outcomes of a randomized controlled study. International Journal of Cardiology 2017;228:173‐9. [DOI: 10.1016/j.ijcard.2016.11.105] - DOI - PubMed
-
- Nosaka K, Miyoshi T, Okawa K, Tsukuda S, Sogo M, Nishibe T, et al. Early initiation of eicosapentaenoic acid and statin treatment is associated with better clinical outcomes than statin alone in patients with acute coronary syndromes: 1‐year outcomes of a randomized controlled study. Journal of the American College of Cardiology 2016;67:573. - PubMed
DO IT 2010 {published and unpublished data}
-
- Aung T, Halsey J, Kromhout D, Gerstein HC, Marchioli R, Tavazzi L, et al. Associations of omega‐3 fatty acid supplement use with cardiovascular disease risks: meta‐analysis of 10 trials involving 77 917 individuals. JAMA Cardiology 2018;3(3):225‐34. [DOI: 10.1001/jamacardio.2017.5205] - DOI - PMC - PubMed
-
- Berstad P, Seljeflot I, Veierod MB, Hjerkinn EM, Arnesen H, Pedersen JI. Supplementation with fish oil affects the association between very long‐chain n‐3 polyunsaturated fatty acids in serum non‐esterified fatty acids and soluble vascular cell adhesion molecule‐1. Clinical Science 2003;105(1):13‐20. - PubMed
DREAM Asbell 2018 {published data only}
-
- NCT02128763. Dry eye assessment and management study (DREAM). clinicaltrials.gov/ct2/show/NCT02128763 (first received 1 May 2014).
ENRGISE 2018 {published data only}
-
- Cauley JA, Manini TM, Lovato L, Talton J, Anton SD, Domanchuk K, et al. ENRGISE Investigators. The enabling reduction of low‐grade inflammation in seniors (ENRGISE) pilot study: screening methods and recruitment results. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 2018;74(8):1296‐302. [DOI: 10.1093/gerona/gly204] - DOI - PMC - PubMed
-
- Manini TM, Anton SD, Beavers DP, Cauley JA, Espeland MA, Fielding RA, et al. ENRGISE pilot study investigators. Eabling reduction of low‐grade inflammation in seniors pilot study: concept, rationale, and design. Journal of the American Geriatrics Society 2017;65:1961‐68. [DOI: 10.1111/jgs.14965] - DOI - PMC - PubMed
-
- NCT02676466. The ENRGISE pilot study (ENRGISE) [The ENRGISE (enabling reduction of low‐grade inflammation in seniors) pilot study]. clinicaltrials.gov/ct2/show/NCT02676466 (first received 8 February 2016).
-
- Pahor M, Anton SD, Beavers DP, Cauley JA, Fielding RA, Kritchevsky SB, et al. for the ENRGISE study investigators. Effect of losartan and fish oil on plasma IL‐6 and mobility in older persons. the ENRGISE pilot randomized clinical trial. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 2018;74(10):1612–19. [DOI: 10.1093/gerona/gly277] - DOI - PMC - PubMed
EPE‐A 2014 {published and unpublished data}
-
- Sanyal AJ, Abdelmalek MF, Suzuki A, Cummings OW, Chojkier M. No significant effects of ethyl‐eicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology 2014;147(2):377‐84.e1. [PUBMED: 24818764] - PubMed
EPIC‐1 2008 {published and unpublished data}
-
- Feagan BG, Sandborn WJ, Mittmann U, Bar‐Meir S, D'Haens G, Bradette M, et al. Omega‐3 free fatty acids for the maintenance of remission in Crohn disease: the EPIC randomized controlled trials. JAMA 2008;299(14):1690‐7. - PubMed
EPIC‐2 2008 {published and unpublished data}
-
- Feagan BG, Sandborn WJ, Mittmann U, Bar‐Meir S, D'Haens G, Bradette M, et al. Omega‐3 free fatty acids for the maintenance of remission in Crohn disease: the EPIC randomized controlled trials. JAMA 2008;229(14):1690‐7. - PubMed
EPOCH 2014 {published and unpublished data}
-
- Danthiir V, Burns NR, Nettelbeck T, Wilson C, Wittert G. The older people, omega‐3, and cognitive health (EPOCH) trial design and methodology: a randomised, double‐blind, controlled trial investigating the effect of long‐chain omega‐3 fatty acids on cognitive ageing and wellbeing in cognitively healthy older adults. Nutrition Journal 2011;10:117. [PUBMED: 22011460] - PMC - PubMed
-
- Danthiir V, Hosking D, Burns NR, Wilson C, Nettelbeck T, Calvaresi E, et al. Cognitive performance in older adults is inversely associated with fish consumption but not erythrocyte membrane n‐3 fatty acids. Journal of Nutrition 2014;144(3):311‐20. [PUBMED: 24353345] - PubMed
Erdogan 2007 {published data only}
-
- Erdogan A, Bayer M, Kollath D, Greiss H, Voss R, Neumann T, et al. Omega AF study: polyunsaturated fatty acids (PUFA) for prevention of atrial fibrillation relapse after successful external cardioversion. Heart Rhythm 2007;4(5):S185‐6.
-
- Heidt MC, Vician M, Stracke SK, Stadlbauer T, Grebe MT, Boening A, et al. Beneficial effects of intravenously administered n‐3 fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: a prospective randomized study. Thoracic and Cardiovascular Surgeon 2009;57:276‐80. [DOI: 10.1055/s-0029-1185301] - DOI - PubMed
FAAT 2005 {published and unpublished data}
-
- Leaf A, Albert CM, Josephson M, Steinhaus D, Kluger J, Kang JX, et al. Prevention of fatal arrhythmias in high‐risk subjects by fish oil n‐3 fatty acid intake. Circulation 2005;112(18):2762‐8. - PubMed
FLAX‐PAD 2013 {published data only (unpublished sought but not used)}
-
- Caligiuri SP, Aukema HM, Ravandi A, Guzman R, Dibrov E, Pierce GN. Flaxseed consumption reduces blood pressure in patients with hypertension by altering circulating oxylipins via an alpha‐linolenic acid‐induced inhibition of soluble epoxide hydrolase. Hypertension 2014;64(1):53‐9. [PUBMED: 24777981] - PubMed
-
- Caligiuri SP, Rodriguez‐Leyva D, Aukema HM, Ravandi A, Weighell W, Guzman R, et al. Dietary flaxseed reduces central aortic blood pressure without cardiac involvement but through changes in plasma oxylipins. Hypertension 2016;68(4):1031‐8. [PUBMED: 27528063] - PubMed
-
- Edel A, Rodriguez‐Leyva D, Weighell W, Vallee R, Aliani M, Guzman R, et al. Flaxseed lignan metabolites elicit antihypertensive effects in PAD patients in the FLAX‐PAD trial. Annals of Nutrition and Metabolism 2013;63:1339.
-
- Edel AL, Rodriguez‐Leyva D, Maddaford TG, Caligiuri SP, Austria JA, Weighell W, et al. Dietary flaxseed independently lowers circulating cholesterol and lowers it beyond the effects of cholesterol‐lowering medications alone in patients with peripheral artery disease. Journal of Nutrition 2015;145(4):749‐57. [PUBMED: 25694068] - PubMed
-
- Leyva DR, Zahradka P, Ramjiawan B, Guzman R, Aliani M, Pierce GN. The effect of dietary flaxseed on improving symptoms of cardiovascular disease in patients with peripheral artery disease: rationale and design of the FLAX‐PAD randomized controlled trial. Contemporary Clinical Trials 2011;32(5):724‐30. [PUBMED: 21616170] - PubMed
FORWARD 2013 {published and unpublished data}
-
- Macchia A, Grancelli H, Varini S, Nul D, Ferrante D, Mariani J, et al. Late‐breaking clinical trials: treatments for prevention of cardiovascular events: a population perspective. Circulation 2012;126:2780‐1.
-
- Macchia A, Grancelli H, Varini S, Nul D, Laffaye N, Mariani J, et al. Omega‐3 fatty acids for the prevention of recurrent symptomatic atrial fibrillation: results of the FORWARD (randomized trial to assess efficacy of PUFA for the maintenance of sinus rhythm in persistent atrial fibrillation) trial. Journal of the American College of Cardiology 2013;61(4):463‐8. [PUBMED: 23265344] - PubMed
-
- Macchia A, Varini S, Grancelli H, Nul D, Laffaye N, Ferrante D, et al. The rationale and design of the FORomegaARD trial: a randomized, double‐blind, placebo‐controlled, independent study to test the efficacy of n‐3 PUFA for the maintenance of normal sinus rhythm in patients with previous atrial fibrillation. American Heart Journal 2009;157(3):423‐7. [PUBMED: 19249410] - PubMed
FOSTAR 2016 {published and unpublished data}
-
- Chen JS, Hill CL, Lester S, Ruediger CD, Battersby R, Jones G, et al. Supplementation with omega‐3 fish oil has no effect on bone mineral density in adults with knee osteoarthritis: a 2‐year randomized controlled trial. Osteoporosis International 2016;27(5):1897‐905. [PUBMED: 26694596] - PubMed
-
- Hill C, Lester SE, Jones G. Response to 'Low‐dose versus high‐dose fish oil for pain reduction and function improvement in patients with knee osteoarthritis' by Chen et al. Annals of the Rheumatic Diseases 2016; Vol. 75, issue 1:e8. [PUBMED: 26662278] - PubMed
-
- Hill CL, March LM, Aitken D, Lester SE, Battersby R, Hynes K, et al. Fish oil in knee osteoarthritis: a randomised clinical trial of low dose versus high dose. Annals of the Rheumatic Diseases 2016;75(1):23‐9. [PUBMED: 26353789] - PubMed
Franzen 1993 {published and unpublished data}
-
- Franzen D. A prospective, randomized, and double‐blind trial on the effect of fish oil on the incidence of restenosis following PTCA. Catheterization and Cardiovascular Diagnosis 1993;28(4):301‐10. - PubMed
-
- Franzen D, Geisel J, Hopp HW, Oette K, Hilger HH. Long‐term effects of low dosage fish oil on serum lipids and lipoproteins [Langzeiteffekte von niedrigdosiertem fischol auf serumlipide und lipoproteine]. Medizinische Klinik 1993;88(3):134‐8. - PubMed
Gill 2012 {published data only}
-
- Gill EA, Chen MA, Paramsothy P, Fish B, Isquith D, Thirumalai A, et al. Omega‐3 fatty acids effects on carotid IMT in metabolic syndrome. Circulation 2014;130:A1269. Abstract no. 12697.
-
- Gill EA, Chen MA, Thirumalai A, Fish B, Paramsothy P. Omega‐3 fatty acids improve dyslipidemia but not inflammatory markers in metabolic syndrome. Journal of Clinical Lipidology 2012;6:278‐9.
GISSI‐HF 2008 {published data only}
-
- Aleksova A, Masson S, Maggioni AP, Lucci D, Fabbri G, Beretta L, et al. N‐3 polyunsaturated fatty acids and atrial fibrillation in patients with chronic heart failure: the GISSI‐HF trial. European Journal of Heart Failure 2013;15(11):1289‐95. - PubMed
-
- Aung T, Halsey J, Kromhout D, Gerstein HC, Marchioli R, Tavazzi L, et al. Associations of omega‐3 fatty acid supplement use with cardiovascular disease risks: meta‐analysis of 10 trials involving 77 917 individuals. JAMA Cardiology 2018;3(3):225‐34. [DOI: 10.1001/jamacardio.2017.5205] - DOI - PMC - PubMed
-
- Canepa M, Temporelli PL, Rossi A, Gonzini L, Nicolosi GL, Staszewsky L, et al. Prevalence and prognostic impact of chronic obstructive pulmonary disease in patients with chronic heart failure. Data from the GISSI‐Heart Failure trial. European Journal of Heart Failure 2016;18:442‐3. - PubMed
-
- Cowie MR, Cure S, Bianic F, McGuire A, Goodall G, Tavazzi L. Cost‐effectiveness of highly purified omega‐3 polyunsaturated fatty acid ethyl esters in the treatment of chronic heart failure: results of Markov modelling in a UK setting. European Journal of Heart Failure 2011;13(6):681‐9. [DOI: 10.1093/eurjhf/hfr023] - DOI - PubMed
-
- Finzi A, Barlera S, Serra DM, Rossi MG, Ruggeri A, Mezzani A, et al. Antiarrhythmic effects of n‐3 PUFA in patients with heart failure and an implantable cardioverter defibrillator in the GISSI‐HF trial. European Heart Journal 2009;30:279.
GISSI‐P 1999 {published data only}
-
- Aung T, Halsey J, Kromhout D, Gerstein HC, Marchioli R, Tavazzi L, et al. Associations of omega‐3 fatty acid supplement use with cardiovascular disease risks: meta‐analysis of 10 trials involving 77 917 individuals. JAMA Cardiology 2018;3(3):225‐34. [DOI: 10.1001/jamacardio.2017.5205] - DOI - PMC - PubMed
-
- Franzosi MG, Brunetti M, Marchioli R, Marfisi RM, Tognoni G, Valagussa F, GISSI‐Prevenzione I. Cost‐effectiveness analysis of n‐3 polyunsaturated fatty acids (PUFA) after myocardial infarction: results from Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto (GISSI)‐Prevenzione Trial. Pharmacoeconomics 2001;19(4):411‐20. - PubMed
-
- GISSI‐Prevenzione Investigators. Dietary supplementation with n‐3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI‐Prevenzione trial. Lancet 1999;354:447‐55. - PubMed
-
- Marchioli R. Treatment with n‐3 polyunsaturated fatty acids after myocardial infarction: results of GISSI‐Prevenzione Trial. European Heart Journal Supplements 2001;3(Suppl D):D85‐D97. - PubMed
-
- Marchioli R, Barzi F, Bomba E, Chieffo C, Gregorio DD, Franzosi MG, et al. Early protection against sudden death by n‐3 polyunsaturated fatty acids after myocardial infarction: time course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)‐Prevenzione. Circulation 2002;105:1897‐903. - PubMed
HARP 1995 {published and unpublished data}
-
- Pasternak RC, Brown LE, Stone PH, Silverman DI, Gibson CM, Sacks FM. Effect of combination therapy with lipid‐reducing drugs in patients with coronary heart disease and "normal" cholesterol levels. A randomized, placebo‐controlled trial. Annals of Internal Medicine 1996;125(7):529‐40. [PUBMED: 8815751] - PubMed
-
- Sacks FM, Pasternak RC, Gibson CM, Rosner B, Stone PH. Effect on coronary atherosclerosis of decrease in plasma cholesterol concentrations in normocholesterolaemic patients. Lancet 1994;344(8931):1182‐6. [PUBMED: 7934538] - PubMed
-
- Sacks FM, Stone PH, Gibson CM, Silverman DI, Rosner B, Pasternak RC. Controlled trial of fish oil for regression of human coronary atherosclerosis. Journal of the American College of Cardiology 1995;25(7):1492‐8. - PubMed
HEARTS 2017 {published data only}
-
- Alfaddagh A, Elajami TK, Ashfaque H, Saleh M, Bistrian BR, Welty FK. Effect of eicosapentaenoic and docosahexaenoic acids added to statin therapy on coronary artery plaque in patients with coronary artery disease: a randomized clinical trial. Journal of the American Heart Association 2017;6(12):e006981. [DOI: 10.1161/JAHA.117.006981] - DOI - PMC - PubMed
-
- Alfaddagh A, Elajami TK, Welty FK. Abstract 16294: Omega‐3 fatty acid added to statin prevents progression of fibrous coronary artery plaque compared to statin alone in patients with coronary artery disease. Circulation 2016;134:A16294.
-
- Alfaddagh A, Mohebali D, Elajami TK, Saleh M, Welty FK. Abstract 17391: Omega‐3 fatty acid index >= 4% prevents progression of coronary artery plaque in non‐diabetic subjects on statin therapy. Circulation 2017;136 (Suppl 1):A17391.
-
- Alfaddagh A, Welty FK. Abstract 17689: Omega‐3 fatty acid supplementation reduces inflammation and improves physical function in patient with coronary artery disease. American Heart Association Conference. 2015:circ.ahajournals.org/content/132/Suppl_3/A17689.short.
-
- Elajami TK, Alfaddagh A, Lakshminarayan D, Soliman M, Chandnani M, Welty FK. Eicosapentaenoic and docosahexaenoic acids attenuate progression of albuminuria in patients with type 2 diabetes mellitus and coronary artery disease. Journal of the American Heart Association 2017;6(7):e004740. [DOI: 10.1161/JAHA.116.004740] - DOI - PMC - PubMed
HERO 2009 {published and unpublished data}
-
- Tan SY. Dietary Manipulation and Weight Management [PhD thesis]. Wollongong, Australia: University of Wollonong, 2010.
-
- Tapsell LC, Batterham MJ, Teuss G, Tan SY, Dalton S, Quick CJ, et al. Long‐term effects of increased dietary polyunsaturated fat from walnuts on metabolic parameters in type II diabetes. European Journal of Clinical Nutrition 2009;63(8):1008‐15. [PUBMED: 19352378] - PubMed
JELIS 2007 {published data only}
-
- Aung T, Halsey J, Kromhout D, Gerstein HC, Marchioli R, Tavazzi L, et al. Associations of omega‐3 fatty acid supplement use with cardiovascular disease risks: meta‐analysis of 10 trials involving 77 917 individuals. JAMA Cardiology 2018;3(3):225‐34. [DOI: 10.1001/jamacardio.2017.5205] - DOI - PMC - PubMed
-
- Itakura H, Yokoyama M, Matsuzaki M, Saito Y, Origasa H, Ishikawa Y, et al. Relationships between plasma fatty acid composition and coronary artery disease. Journal of Atherosclerosis and Thrombosis 2011;18(2):99‐107. [PUBMED: 21099130] - PubMed
-
- Itakura H, Yokoyama M, Matsuzaki M, Saito Y, Origasa H, Ishikawa Y, et al. The change in low‐density lipoprotein cholesterol concentration is positively related to plasma docosahexaenoic acid but not eicosapentaenoic acid. Journal of Atherosclerosis and Thrombosis 2012;19(7):673‐9. [PUBMED: 22653220] - PubMed
Kumar 2012 {published data only (unpublished sought but not used)}
Kumar 2013 {published data only (unpublished sought but not used)}
-
- Kumar S, Sutherland F, Stevenson I, Lee JM, Garg ML, Sparks PB. Effects of long‐term omega‐3 polyunsaturated fatty acid supplementation on paroxysmal atrial tachyarrhythmia burden in patients with implanted pacemakers: results from a prospective randomised study. International Journal of Cardiology 2013;168(4):3812‐7. [PUBMED: 23890856] - PubMed
Lorenz‐Meyer 1996 {published and unpublished data}
-
- Lorenz‐Meyer H, Bauer P, Nicolay C, Schulz B, Purrmann J, Fleig WE, et al. Omega‐3 fatty acids and low carbohydrate diet for maintenance of remission in Crohn's disease. A randomized controlled multicenter trial. Scandinavian Journal of Gastroenterology 1996;31(8):778‐85. - PubMed
MAPT 2017 {published data only}
-
- Andrieu S, Guyonnet S, Coley N, Cantet C, Bonnefoy M, Bordes S, et al. Effect of long‐term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo‐controlled trial. Lancet Neurology 2017;16:377‐89. [DOI: 10.1016/S1474-4422(17)30040-6] - DOI - PubMed
-
- Barreto PdS, Rolland Y, Cesari M, Dupuy C, Andrieu S, Vellas B, for the MAPT Study Group. Effects of multidomain lifestyle intervention, omega‐3 supplementation or their combination on physical activity levels in older adults: secondary analysis of the Multidomain Alzheimer Preventive Trial (MAPT) randomised controlled trial. Age and Ageing 2017;47(2):281‐8. [DOI: 10.1093/ageing/afx164] - DOI - PubMed
-
- Carrie I, Kan GA, Gillette‐Guyonnet S, Andrieu S, Dartigues JF, Touchon J, et al. Recruitment strategies for preventive trials. The MAPT study (MultiDomain Alzheimer Preventive Trial). Journal of Nutrition, Health & Aging 2012;16(4):355‐9. - PubMed
-
- Delrieu J, Payoux P, Hitzel A, Peiffer S, Abellan Van Kan G, Gillette S, et al. Multidomain Alzheimer's disease preventive trial: florbetapir ancillary study. Alzheimer's and Dementia 2011;1:S419.
MARGARIN 2002 {published data only (unpublished sought but not used)}
-
- Bemelmans WJ, Broer J, Vries JH, Hulshof KF, May JF, Meyboom‐De Jong B. Impact of Mediterranean diet education versus posted leaflet on dietary habits and serum cholesterol in a high risk population for cardiovascular disease. Public Health Nutrition 2000;3(3):273‐83. - PubMed
-
- Bemelmans WJ, Broer J, Feskens EJ, Smit AJ, Muskiet AJ, Lefrandt JD, et al. Effect of an increased intake of alpha‐linolenic acid and group nutritional education on cardiovascular risk factors: the Mediterranean alpha‐linolenic enriched Groningen dietary intervention (MARGARIN) study. American Journal of Clinical Nutrition 2002;75:221‐7. - PubMed
-
- Bemelmans WJ, Lefrandt JD, Feskens EJ, Broer J, Tervaert JW, May JF, et al. Change in saturated fat intake is associated with progression of carotid and femoral intima‐media thickness, and with levels of soluble intercellular adhesion molecule‐1. Atherosclerosis 2002;163(1):113‐20. - PubMed
-
- Bemelmans WJ, Lefrandt JD, Feskens EJ, Haelst PL, Broer J, Meyboom‐de Jong B, et al. Increased alpha‐linolenic acid intake lowers C‐reactive protein, but has no effect on markers of atherosclerosis. European Journal of Clinical Nutrition 2004;58(7):1083‐9. - PubMed
-
- Bemelmans WJ, Muskiet FA, Feskens EJ, Vries JH, Broer J, May JF, et al. Associations of alpha‐linolenic acid and linoleic acid with risk factors for coronary heart disease. European Journal of Clinical Nutrition 2000;54(12):865‐71. - PubMed
MARINA 2011 {published and unpublished data}
-
- AlSaleh A, Maniou Z, Lewis FJ, Hall WL, Sanders TA, O'Dell SD. Interaction between a CSK gene variant and fish oil intake influences blood pressure in healthy adults. Journal of Nutrition 2014;144(3):267‐72. [PUBMED: 24401815] - PubMed
-
- Alsaleh A, Crepostnaia D, Maniou Z, Lewis FJ, Hall WL, Sanders TA, et al. Adiponectin gene variant interacts with fish oil supplementation to influence serum adiponectin in older individuals. Journal of Nutrition 2013;143(7):1021‐7. [PUBMED: 23658423] - PubMed
-
- Pinto AM, Hall WL, Sanders TAB. Effect of low doses of long chain n‐3 PUFA intake on daytime heart rate variability: results from the MARINA study. European Journal of Preventive Cardiology 2015;1:S146.
MENU 2016 {published data only (unpublished sought but not used)}
-
- Le T, Flatt SW, Natarajan L, Pakiz B, Quintana EL, Heath DD, et al. Effects of diet composition and insulin resistance status on plasma lipid levels in a weight loss intervention in women. Journal of the American Heart Association 2016;5(1):e002771. [DOI: 10.1161/JAHA.115.002771] - DOI - PMC - PubMed
-
- Rock CL, Flatt SW, Pakiz B, Quintana EL, Heath DD, Rana BK, et al. Effects of diet composition on weight loss, metabolic factors and biomarkers in a 1‐year weight loss intervention in obese women examined by baseline insulin resistance status. Metabolism 2016;65(11):1605‐13. [DOI: 10.1016/j.metabol.2016.07.008] - DOI - PMC - PubMed
Mita 2007 {published data only}
-
- Mita T, Watada H, Ogihara T, Nomiyama T, Ogawa O, Kinoshita J, et al. Eicosapentaenoic acid reduces the progression of carotid intima‐media thickness in patients with type 2 diabetes. Atherosclerosis 2007;191(1):162‐7. - PubMed
NAT2 2013 {published data only}
-
- Merle BM, Benlian P, Puche N, Bassols A, Delcourt C, Souied EH. Circulating omega‐3 fatty acids and neovascular age‐related macular degeneration. Investigative Ophthalmology & Visual Science 2014;55(3):2010‐9. [PUBMED: 24557349] - PubMed
-
- Souied EH, Delcourt C, Querques G, Bassols A, Merle B, Zourdani A, et al. Oral docosahexaenoic acid in the prevention of exudative age‐related macular degeneration: the nutritional AMD treatment 2 study. Ophthalmology 2013;120(8):1619‐31. [PUBMED: 23395546] - PubMed
Nodari 2011 AF {published data only}
-
- Nodari S, Triggiani M, Campia U, Manerba A, Milesi G, Cesana BM, et al. N‐3 polyunsaturated fatty acids in the prevention of atrial fibrillation recurrences after electrical cardioversion: a prospective, randomized study. Circulation 2011;124(10):1100‐6. [PUBMED: 21844082] - PubMed
Nodari 2011 HF {published and unpublished data}
-
- NCT01223703. PUFAs and left ventricular function in heart failure (CS‐PUFA‐02) [Effects of n‐3 polyunsaturated fatty acids (PUFAs) on left ventricular function and functional capacity in patients with dilated cardiomyopathy]. clinicaltrials.gov/ct2/show/NCT01223703 (first received 19 October 2010).
-
- Nodari S, Triggiani M, Berlinghieri N, Milesi G, Foresti A, Gheorghiade M, et al. Effects of n‐3 polyunsaturated fatty acids on left ventricular function and functional capacity in heart failure patients. European Heart Journal 2010;31:850.
-
- Nodari S, Triggiani M, Campia U, Manerba A, Milesi G, Cesana BM, et al. Effects of n‐3 polyunsaturated fatty acids on left ventricular function and functional capacity in patients with dilated cardiomyopathy. Journal of the American College of Cardiology 2011;57(7):870‐9. - PubMed
-
- Nodari S, Triggiani M, Campia U, Zhao L, Manerba A, Milesi G, et al. Plasma levels of n‐3 polyunsaturated fatty acids and risk of hospitalization in patients with non‐ischemic cardiomyopathy. Circulation 2012;126(21 Suppl 1):A17431.
Norouzi 2014 {published data only}
-
- Norouzi Javidan A, Sabour H, Latifi S, Abrishamkar M, Soltani Z, Shidfar F, et al. Does consumption of polyunsaturated fatty acids influence on neurorehabilitation in traumatic spinal cord‐injured individuals? A double‐blinded clinical trial. Spinal Cord 2014;52(5):378‐82. [PUBMED: 24637568] - PubMed
-
- Sabour H, Norouzi Javidan A, Latifi S, Shidfar F, Heshmat R, Emami Razavi SH, et al. Omega‐3 fatty acids' effect on leptin and adiponectin concentrations in patients with spinal cord injury: a double‐blinded randomized clinical trial. Journal of Spinal Cord Medicine 2015;38(5):599‐606. [PUBMED: 25096818] - PMC - PubMed
Norwegian 1968 {published data only}
-
- Natvig H. The effect of unsaturated fatty acids on the incidence of coronary infarction, etc [Effekten av umettede fettsyrer hyppigheten av hjerteinfarkt M.M.]. Tidsskrift for Den Norske Laegeforening 1967;87(11):1033‐41. - PubMed
-
- Natvig H, Borchgrevink CF, Dedichen J, Owren PA, Schiotz EH, Westlund K. A controlled trial of the effect of linolenic acid on incidence of coronary heart disease. The Norwegian vegetable oil experiment of 1965‐66. Scandinavian Journal of Clinical and Laboratory Investigation 1968;105(Suppl):1‐20. - PubMed
Nutristroke 2009 {published data only}
-
- Garbagnati F, Cairella G, Martino A, Multari M, Scognamiglio U, Venturiero V, et al. Is antioxidant and n‐3 supplementation able to improve functional status in post‐stroke patients? Results from the Nutristroke Trial. Cerebrovascular Diseases 2009;27(4):375‐83. [DOI: 10.1159/000207441] - DOI - PubMed
Nye 1990 {published data only}
-
- Ilsey CD, Nye ER, Sutherland W, Ram J, Ablett MB. Randomised placebo controlled trial of MAXEPA and aspirin/persantin after successful coronary angioplasty. Australian & New Zealand Journal of Medicine 1987;17:559.
-
- Nye ER, Ablett MB, Robertson MC, Ilsley CD, Sutherland WH. Effect of eicosapentaenoic acid on restenosis rate, clinical course and blood lipids in patients after percutaneous transluminal coronary angioplasty. Australian and New Zealand Journal of Medicine 1990;20(4):549‐52. - PubMed
OFAMI 2001 {published and unpublished data}
-
- Aarsetoy H, Brugger‐Andersen T, Hetland O, Grundt H, Nilsen DW. Long term influence of regular intake of high dose n‐3 fatty acids on CD40‐ligand, pregnancy‐associated plasma protein A and matrix metalloproteinase‐9 following acute myocardial infarction. Thrombosis and Haemostasis 2006;95(2):329‐36. - PubMed
-
- Grundt H, Hetland O, Nilsen DW. Changes in tissue factor and activated factor XII following an acute myocardial infarction were uninfluenced by high doses of n‐3 polyunsaturated fatty acids. Thrombosis and Haemostasis 2003;89(4):752‐9. - PubMed
-
- Grundt H, Nilsen DW, Hetland O, Mansoor MA. Clinical outcome and atherothrombogenic risk profile after prolonged wash‐out following long‐term treatment with high doses of n‐3 PUFAs in patients with an acute myocardial infarction. Clinical Nutrition 2004;23(4):491‐500. - PubMed
-
- Grundt H, Nilsen DW, Mansoor MA, Hetland O, Nordoy A. Reduction in homocysteine by n‐3 polyunsaturated fatty acids after 1 year in a randomised double‐blind study following an acute myocardial infarction: no effect on endothelial adhesion properties. Pathophysiology of Haemostasis and Thrombosis 2003;33(2):88‐95. - PubMed
-
- Grundt H, Nilsen DW, Mansoor MA, Nordoy A. Increased lipid peroxidation during long‐term intervention with high doses of n‐3 fatty acids (PUFAs) following an acute myocardial infarction. European Journal of Clinical Nutrition 2003;57(6):793‐800. - PubMed
OMEGA 2009 {published and unpublished data}
-
- Aung T, Halsey J, Kromhout D, Gerstein HC, Marchioli R, Tavazzi L, et al. Associations of omega‐3 fatty acid supplement use with cardiovascular disease risks: meta‐analysis of 10 trials involving 77 917 individuals. JAMA Cardiology 2018;3(3):225‐34. [DOI: 10.1001/jamacardio.2017.5205] - DOI - PMC - PubMed
-
- Rauch B, Schiele R, Schneider S, Diller F, Victor N, Gohlke H, et al. OMEGA, a randomized, placebo‐controlled trial to test the effect of highly purified omega‐3 fatty acids on top of modern guideline‐adjusted therapy after myocardial infarction. Circulation 2010;122(21):2152‐9. [DOI: 10.1161/CIRCULATIONAHA.110.948562] - DOI - PubMed
-
- Rauch B, Schiele R, Schneider S, Gohlke H, Diller F, Gottwik M, et al. Highly purified omega‐3 fatty acids for secondary prevention of sudden cardiac death after myocardial infarction‐aims and methods of the OMEGA‐study. Cardiovascular Drugs Therapy 2006;20(5):365‐75. [DOI: 10.1007/s10557-006-0495-6] - DOI - PubMed
-
- Zimmer R, Riemer T, Rauch B, Schneider S, Schiele R, Gohlke H, et al. Effects of 1‐year treatment with highly purified omega‐3 fatty acids on depression after myocardial infarction: results from the OMEGA trial. Journal of Clinical Psychiatry 2013;74(11):e1037‐45. [PUBMED: 24330904] - PubMed
OPAL 2010 {published and unpublished data}
-
- Dangour AD, Allen E, Elbourne D, Fasey N, Fletcher AE, Hardy P, et al. Effect of 2‐y n‐3 long‐chain polyunsaturated fatty acid supplementation on cognitive function in older people: a randomized, double‐blind, controlled trial. American Journal of Clinical Nutrition 2010;91(6):1725‐32. - PubMed
-
- Dangour AD, Allen E, Elbourne D, Fletcher A, Richards M, Uauy R. Fish consumption and cognitive function among older people in the UK: baseline data from the OPAL study. Journal of Nutrition, Health & Aging 2009;13(3):198‐202. - PubMed
-
- Dangour AD, Allen E, Elbourne D, Fletcher AE, Neveu MM, Uauy R, et al. N‐3 fatty acids and retinal function. Ophthalmology 2013;120(3):643. [DOI: ] - PubMed
-
- Dangour AD, Clemens F, Elbourne D, Fasey N, Fletcher AE, Hardy P, et al. A randomised controlled trial investigating the effect of n‐3 long‐chain polyunsaturated fatty acid supplementation on cognitive and retinal function in cognitively healthy older people: the Older People And n‐3 Long‐chain polyunsaturated fatty acids (OPAL) study protocol [ISRCTN72331636]. Nutrition Journal 2006;5:20. - PMC - PubMed
-
- ISRCTN72331636. The OPAL Study: older people and n‐3 long‐chain polyunsaturated fatty acids. www.isrctn.com/ISRCTN72331636 (first received 27 April 2004). [ISRCTN72331636]
ORIGIN 2012 {published data only}
-
- Aung T, Halsey J, Kromhout D, Gerstein HC, Marchioli R, Tavazzi L, et al. Associations of omega‐3 fatty acid supplement use with cardiovascular disease risks: meta‐analysis of 10 trials involving 77 917 individuals. JAMA Cardiology 2018;3(3):225‐34. [DOI: 10.1001/jamacardio.2017.5205] - DOI - PMC - PubMed
-
- Bordeleau L, Yakubovich N, Dagenais G, Rosenstock J, Ryden LE, Spinas G, et al. Cancer outcomes in patients with dysglycemia on basal insulin: results of the Origin trial. Diabetes 2013;62:A72.
-
- Bordeleau L, Yakubovich N, Dagenais GR, Rosenstock J, Probstfield J, Chang Yu P, et al. The association of basal insulin glargine and/or n‐3 fatty acids with incident cancers in patients with dysglycemia. Diabetes Care 2014;37(5):1360‐6. - PubMed
-
- Lonn EM, Bosch J, Diaz R, Lopez‐Jaramillo P, Ramachandran A, Hancu N, et al. Effect of insulin glargine and n‐3FA on carotid intima‐media thickness in people with dysglycemia at high risk for cardiovascular events: the glucose reduction and atherosclerosis continuing evaluation study (ORIGIN‐GRACE). Diabetes Care 2013;36(9):2466‐74. - PMC - PubMed
-
- Maggioni AP, Fabbri G, Bosch J, Dyal L, Ryden LE, Gerstein HC, et al. Effects of n‐3 fatty acids on long‐term outcomes of high risk patients with type 2 diabetes mellitus or IGF/IGT with a recent myocardial infarction. European Heart Journal 2013;34:352.
ORL 2013 {published data only}
-
- Tatsuno I, Saito Y, Kudou K, Ootake J. Long‐term safety and efficacy of TAK‐085 in Japanese subjects with hypertriglyceridemia undergoing lifestyle modification: the omega‐3 fatty acids randomized long‐term (ORL) study. Journal of Clinical Lipidology 2013;7(6):615‐25. [PUBMED: 24314359] - PubMed
-
- Tatsuno IT, Saito YS, Kudou KK, Otake JO, Minamide YM. Effects of long‐term treatment with omega‐3 polyunsaturated fatty acids (Lotriga) on atherogenic lipoproteins in hypertriglyceridemia: results from a phase 3 randomized, open‐label, 1‐year study. European Heart Journal 2013;34:768.
Özaydin 2011 {published data only}
-
- Özaydin M, Erdoğan D, Tayyar S, Uysal BA, Doğan A, Içli A, et al. N‐3 polyunsaturated fatty acids administration does not reduce the recurrence rates of atrial fibrillation and inflammation after electrical cardioversion: a prospective randomized study. Anadolu Kardiyoloji Dergisi 2011;11(4):305‐9. [DOI: 10.5152/akd.2011.080] - DOI - PubMed
Proudman 2015 {published and unpublished data}
-
- Proudman S, Spargo L, Hall C, McWilliams L, Lee A, Maureen R, et al. Fish oil in rheumatoid arthritis: a randomised, double blind trial comparing high dose with low dose. Internal Medicine Journal 2012;42(Suppl 1):2‐3.
-
- Proudman SM, Cleland LG, Metcalf RG, Sullivan TR, Spargo LD, James MJ. Plasma n‐3 fatty acids and clinical outcomes in recent‐onset rheumatoid arthritis. British Journal of Nutrition 2015;114(6):885‐90. [PUBMED: 26283657] - PubMed
-
- Proudman SM, James MJ, Spargo LD, Metcalf RG, Sullivan TR, Rischmueller M, et al. Fish oil in recent onset rheumatoid arthritis: a randomised, double‐blind controlled trial within algorithm‐based drug use. Annals of the Rheumatic Diseases 2015;74(1):89‐95. [PUBMED: 24081439] - PubMed
Puri 2005 {published data only}
-
- Puri BK, Leavitt BR, Hayden MR, Ross CA, Rosenblatt A, Greenamyre JT, et al. Ethyl‐EPA in Huntington disease: a double‐blind, randomized, placebo‐controlled trial. Neurology 2005;65(2):286‐92. - PubMed
Raitt 2005 {published data only}
-
- Raitt MH, Connor WE, Morris C, Kron J, Halperin B, Chugh SS, et al. Fish oil supplementation and risk of ventricular tachycardia and ventricular fibrillation in patients with implantable defibrillators: a randomized controlled trial. JAMA 2005;293(23):2884‐91. - PubMed
Ramirez‐Ramirez 2013 {published data only}
-
- Ramirez‐Ramirez V, Macias‐Islas MA, Ortiz GG, Pacheco‐Moises F, Torres‐Sanchez ED, Sorto‐Gomez TE, et al. Efficacy of fish oil on serum of TNF α , IL‐1 β , and IL‐6 oxidative stress markers in multiple sclerosis treated with interferon beta‐1b. Oxidative Medicine and Cellular Longevity 2013;2013:709493. [DOI: 10.1155/2013/709493] - DOI - PMC - PubMed
REDUCE‐IT 2019 {published data only}
-
- Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Effects of icosapent ethyl on total ischemic events: from REDUCE‐IT. Journal of the American College of Cardiology 2019;73(22):2791‐802. - PubMed
-
- NCT01492361. A study of AMR101 to evaluate its ability to reduce cardiovascular events in high risk patients with hypertriglyceridemia and on statin. The primary objective is to evaluate the effect of 4 g/day AMR101 for preventing the occurrence of a first major cardiovascular event (REDUCE‐IT). clinicaltrials.gov/ct2/show/NCT01492361 (first received 15 December 2011).
Reed 2014 {published and unpublished data}
Risk & Prevention 2013 {published and unpublished data}
-
- Aung T, Halsey J, Kromhout D, Gerstein HC, Marchioli R, Tavazzi L, et al. Associations of omega‐3 fatty acid supplement use with cardiovascular disease risks: meta‐analysis of 10 trials involving 77 917 individuals. JAMA Cardiology 2018;3(3):225‐34. [DOI: 10.1001/jamacardio.2017.5205] - DOI - PMC - PubMed
-
- Rischio and Prevenzione Investigators. Efficacy of n‐3 polyunsaturated fatty acids and feasibility of optimizing preventive strategies in patients at high cardiovascular risk: rationale, design and baseline characteristics of the Rischio and Prevenzione study, a large randomised trial in general practice. Trials 2010;11(1):68. - PMC - PubMed
-
- Risk and Prevention Study Collaborative Group, Roncaglioni MC, Tombesi M, Avanzini F, Barlera S, Caimi V, et al. N‐3 fatty acids in patients with multiple cardiovascular risk factors. New England Journal of Medicine 2013;368(19):1800‐8. - PubMed
-
- Visentin G, Risk & Prevention Study Group. Towards evidence‐based practice via practice‐based evidence: the Italian experience. Family Practice 2008;25(Suppl 1):i71‐4. - PubMed
Rossing 1996 {published and unpublished data}
-
- Myrup B, Rossing P, Jensen T, Parving H‐H, Holmer G, Gram J, et al. Lack of effect of fish oil supplementation on coagulation and transcapillary escape rate of albumin in insulin‐dependent diabetic patients with diabetic nephropathy. Scandinavian Journal of Clinical & Laboratory Investigation 2001;61(5):349‐56. - PubMed
-
- Rossing P, Hansen BV, Nielsen FS, Myrup B, Holmer G, Parving HH. Fish oil in diabetic nephropathy. Diabetes Care 1996;19(11):1214‐9. - PubMed
Sandhu 2016 {published data only}
-
- Sandhu N, Schetter SE, Liao J, Hartman TJ, Richie JP, McGinley J, et al. Influence of obesity on breast density reduction by omega‐3 fatty acids: evidence from a randomized clinical trial. Cancer Prevention Research 2016;9(4):275‐82. [PUBMED: 26714774] - PubMed
-
- Signori C, DuBrock C, Richie JP, Prokopczyk B, Demers LM, Hamilton C, et al. Administration of omega‐3 fatty acids and Raloxifene to women at high risk of breast cancer: interim feasibility and biomarkers analysis from a clinical trial. European Journal of Clinical Nutrition 2012;66(8):878‐84. [PUBMED: 22669332] - PubMed
SCIMO 1999 {published and unpublished data}
-
- Angerer P, Kothny W, Stork S, Schacky C. Effect of dietary supplementation with omega‐3 fatty acids on progression of atherosclerosis in carotid arteries. Cardiovascular Research 2002;54(1):183‐90. - PubMed
-
- Schacky C, Angerer P, Kothny W, Theisen K, Mudra H. The effect of dietary omega‐3 fatty acids on coronary atherosclerosis. A randomized, double‐blind, placebo‐controlled trial. Annals of Internal Medicine 1999;130(7):554‐62. - PubMed
-
- Schacky C, Baumann K, Angerer P. The effect of n‐3 fatty acids on coronary atherosclerosis: results from SCIMO, an angiographic study, background and implications. Lipids 2001;36(Suppl):S99‐102. - PubMed
seAFOod Hull 2018 {published data only}
-
- Hull MA, Sandell AC, Montgomery AA, Logan RF, Clifford GM, Rees CJ, et al. A randomized controlled trial of eicosapentaenoic acid and/or aspirin for colorectal adenoma prevention during colonoscopic surveillance in the NHS Bowel Cancer Screening Programme (The seAFOod Polyp Prevention Trial): study protocol for a randomized controlled trial. Trials [Electronic Resource] 2013;14:237. - PMC - PubMed
-
- Hull MA, Sprange K, Hepburn T, Tan W, Shafayat A, Rees CJ, et al. Eicosapentaenoic acid and aspirin, alone and in combination, for the prevention of colorectal adenomas (seAFOod Polyp Prevention trial): a multicentre, randomised, double‐blind, placebo‐controlled, 2x2 factorial trial. Lancet 2018;392(10164):2583‐94. [DOI: 10.1016/S0140-6736(18)31775-6] - DOI - PMC - PubMed
Shinto 2014 {published data only}
-
- NCT00090402. Fish oil and alpha lipoic acid in treating Alzheimer's Disease [Fish Oil and Alpha Lipoic Acid in Mild Alzheimer's Disease]. clinicaltrials.gov/ct2/show/NCT00090402 (first received 24 August 2004). [CENTRAL: NCT00090402]
SHOT 1996 {published and unpublished data}
-
- Eritsland J, Arnesen H, Berg K, Seljeflot I, Abdelnoor M. Serum Lp(a) lipoprotein levels in patients with coronary artery disease and the influence of long‐term n‐3 fatty acid supplementation. Scandinavian Journal of Clinical and Laboratory Investigation 1995;55(4):295‐300. - PubMed
-
- Eritsland J, Arnesen H, Gronseth K, Fjeld NB, Abdelnoor M. Effect of dietary supplementation with n‐3 fatty acids on coronary artery bypass graft patency. American Journal of Cardiology 1996;77(1):31‐6. - PubMed
-
- Eritsland J, Arnesen H, Gronseth K, Fjeld NB, Abdelnoor M. Effect of supplementation with n‐3 fatty acids on graft patency in patients undergoing coronary artery bypass operation. Results from SHOT study. European Heart Journal 1994;15:29.
-
- Eritsland J, Arnesen H, Seljeflot I, Hostmark AT. Long‐term metabolic effects of n‐3 polyunsaturated fatty acids in patients with coronary artery disease. American Journal of Clinical Nutrition 1995;61(4):831‐6. - PubMed
-
- Eritsland J, Arnesen H, Seljeflot I, Kierulf P. Long‐term effects of n‐3 polyunsaturated fatty acids on haemostatic variables and bleeding episodes in patients with coronary artery disease. Blood Coagulation & Fibrinolysis 1995;6(1):17‐22. - PubMed
Sianni 2013 {published data only}
-
- Sianni A, Matsoukis I, Ganotopoulou A, Paraskevas P, Asimis A, Tsivilis N, et al. Effect of omega 3 fatty acids in patients with hypertension and atrial fibrillation. Clinical Nutrition 2013;32:S70‐1.
SMART 2013 {published and unpublished data}
-
- Tapsell LC, Batterham MJ, Charlton KE. Effect of dietary restriction and n‐3 PUFA supplementation on insulin resistance in obese adults. FASEB Journal 2010;24:733.9.
-
- Zhang Q, O'Shea JE, Thorne RL, Tapsell LC, Batterham M, Charlton KE. Baseline characteristics of volunteers in the smart clinical trial: associations between habitual physical activity and lifestyle disease risk factors. Nutrition and Dietetics 2010;67(Suppl 1):67‐8.
SOFA 2006 {published and unpublished data}
-
- Brouwer IA, Katan MB, Schouten EG, Camm AJ, Hauer RN, Wever EF, et al. Rationale and design of a clinical trial on n‐3 fatty acids and cardiac arrhythmia (SOFA). Annals of Nutrition & Metabolism 2001;45(Suppl 1):79. - PubMed
-
- Brouwer IA, Zock PL, Camm AJ, Böcker D, Hauer RN, Wever EF, et al. Effect of fish oil on ventricular tachyarrhythmia and death in patients with implantable cardioverter defibrillators: the study on omega‐3 fatty acids and ventricular arrhythmia (SOFA) randomized trial. JAMA 2006;295(22):2613‐9. - PubMed
-
- Brouwer IA, Zock PL, Wever EF, Hauer RN, Camm AJ, Bocker D, et al. Rationale and design of a randomised controlled clinical trial on supplemental intake of n‐3 fatty acids and incidence of cardiac arrhythmia: SOFA. European Journal of Clinical Nutrition 2003;57:1323‐30. - PubMed
Sofi 2010 {published data only}
-
- Sofi F, Giangrandi I, Cesari F, Corsani I, Abbate R, Gensini GF, et al. Effects of a 1‐year dietary intervention with n‐3 polyunsaturated fatty acid‐enriched olive oil on non‐alcoholic fatty liver disease patients: a preliminary study. International Journal of Food Sciences and Nutrition 2010;61(8):792‐802. [PUBMED: 20465434] - PubMed
SU.FOL.OM3 2010 {published and unpublished data}
-
- Ahluwalia N, Blacher J, Szabo De Edelenyi F, Faure P, Julia C, Hercberg S, et al. Prognostic value of multiple emerging biomarkers in cardiovascular risk prediction in patients with stable cardiovascular disease. Atherosclerosis 2013;228(2):478‐84. - PubMed
-
- Andreeva VA, Galan P, Torres M, Julia C, Hercberg S, Kesse‐Guyot E. Supplementation with B vitamins or n‐3 fatty acids and depressive symptoms in cardiovascular disease survivors: ancillary findings from the SUpplementation with FOLate, vitamins B‐6 and B‐12 and/or OMega‐3 fatty acids (SU.FOL.OM3) randomized trial. American Journal of Clinical Nutrition 2012;96(1):208‐14. - PubMed
-
- Andreeva VA, Kesse‐Guyot E, Barberger‐Gateau P, Fezeu L, Hercberg S, Galan P. Cognitive function after supplementation with B vitamins and long‐chain omega‐3 fatty acids: ancillary findings from the SU.FOL.OM3 randomized trial. American Journal of Clinical Nutrition 2011;94(1):278‐86. - PubMed
-
- Andreeva VA, Touvier M, Kesse‐Guyot E, Julia C, Galan P, Hercberg S. B vitamin and/or omega‐3 fatty acid supplementation and cancer: ancillary findings from the supplementation with folate, vitamins B6 and B12, and/or omega‐3 fatty acids (SU.FOL.OM3) randomized trial. Archives of Internal Medicine 2012;172(7):540‐7. - PubMed
Tande 2016 {published and unpublished data}
-
- Tande KS, Vo TD, Lynch BS. Clinical safety evaluation of marine oil derived from Calanus finmarchicus. Regulatory Toxicology and Pharmacology: RTP 2016;80:25‐31. [PUBMED: 27233921] - PubMed
THIS DIET 2008 {published and unpublished data}
-
- Packard DP, Milton JE, Shuler LA, Short RA, Tuttle KR. Implications of chronic kidney disease for dietary treatment of cardiovascular disease. Journal of Renal Nutrition 2006;16(3):259‐68. - PubMed
-
- Tuttle KR, Shuler LA, Packard DP, Milton JE, Daratha KB, Bibus DM, et al. Comparison of low‐fat versus Mediterranean‐style dietary intervention after first myocardial infarction (from The Heart Institute of Spokane Diet Intervention and Evaluation Trial). American Journal of Cardiology 2008;101(11):1523‐30. [PUBMED: 18489927] - PubMed
VITAL 2019 {published data only}
-
- Gold DR, Litonjua AA, Carey VJ, Manson JE, Buring JE, Lee IM, et al. Lung VITAL: rationale, design, and baseline characteristics of an ancillary study evaluating the effects of vitamin D and/or marine omega‐3 fatty acid supplements on acute exacerbations of chronic respiratory disease, asthma control, pneumonia and lung function in adults. Contemporary Clinical Trials 2016;47:185‐95. - PMC - PubMed
-
- Gold DR, Luttmann‐Gibson H, Litonjua AA, Friedenberg G, Gordon D, Lee IM, et al. Baseline chronic obstructive pulmonary disease in the lung vitamin D and omega‐3 trial. American Journal of Respiratory and Critical Care Medicine 2014;189:D44 COPD.
-
- Kang JH, Grodstein F, Manson JAE. Cognitive substudy of the vitamin d and omega‐3 trial (VITAL‐COG): design of a large randomized trial of omega‐3 and vitamin D supplements in relation to cognitive change. Alzheimer's & Dementia 2015;1:608.
-
- LeBoff MS, Yue AY, Copeland T, Cook NR, Buring JE, Manson JE. VITAL‐Bone Health: rationale and design of two ancillary studies evaluating the effects of vitamin D and/or omega‐3 fatty acid supplements on incident fractures and bone health outcomes in the VITamin D and OmegA‐3 TriaL (VITAL). Contemporary Clinical Trials 2015;41:259‐68. - PMC - PubMed
WAHA 2016 {published and unpublished data}
-
- Bitok E, Jaceldo‐Siegl K, Rajaram S, Serra‐Mir M, Roth I, Feitas‐Simoes T, et al. Favourable nutrient intake and displacement with long‐term walnut supplementation among elderly: results of a randomised trial. British Journal of Nutrition 2017;118(3):201‐9. [DOI: 10.1017/S0007114517001957] - DOI - PubMed
-
- Bitok E, Rajaram S, Ros E. Does a daily walnut supplement given for a year result in body weight gain?. FASEB Journal 2016; Vol. 30:1157.5.
-
- Huey L, Bitok E, Kazzi N. Dietary compliance of walnut or no walnut intake in a 1‐year randomized intervention trial among free‐living elderly in the Walnuts and Healthy Aging Study (WAHA). FASEB Journal 2016; Vol. 30:1157.10.
-
- Ros E, Rajaram S, Sala‐Vila A, Serra‐Mir M, Vals‐Pedret C, Cofan M, et al. Effect of a 1‐year walnut supplementation on blood lipids among older individuals: findings from the Walnuts and Healthy Aging (WAHA) study. FASEB Journal 2016; Vol. 30, issue 1 Suppl:293.4.
Weinstock‐Guttman 2005 {published data only}
-
- Weinstock‐Guttman B, Baier M, Park Y, Feichter J, Lee‐Kwen P, Gallagher E, et al. Low fat dietary intervention with n‐3 fatty acid supplementation in multiple sclerosis patients. Prostaglandins, Leukotrienes and Essential Fatty Acids 2005;73:397‐404. [DOI: 10.1016/j.plefa.2005.05.024] - DOI - PubMed
WELCOME 2015 {published and unpublished data}
-
- Bhatia L, Scorletti E, Curzen N, Clough GF, Calder PC, Byrne CD. Improvement in non‐alcoholic fatty liver disease severity is associated with a reduction in carotid intima‐media thickness progression. Atherosclerosis 2016;246:13‐20. [PUBMED: 26748347] - PubMed
-
- Byrne CD, Targher G. Ectopic fat, insulin resistance, and nonalcoholic fatty liver disease: implications for cardiovascular disease. Arteriosclerosis, Thrombosis, and Vascular Biology 2014;34(6):1155‐61. [PUBMED: 24743428] - PubMed
-
- Byrne CD, Targher G. Time to replace assessment of liver histology with MR‐based imaging tests to assess efficacy of interventions for nonalcoholic fatty liver disease. Gastroenterology 2016; Vol. 150, issue 1:7‐10. [PUBMED: 26602219] - PubMed
-
- Clough GF, McCormick KG, Scorletti E, Bhatia L, Calder PC, Griffin MJ, et al. Higher body fat percentage is associated with enhanced temperature perception in NAFLD: results from the randomised Wessex evaluation of fatty liver and cardiovascular markers in NAFLD with Omacor therapy trial (WELCOME) trial. Diabetologia 2016;59(7):1422‐9. [PUBMED: 27106721] - PubMed
-
- Hodson L, Bhatia L, Scorletti E, Smith DE, Jackson NC, Shojaee‐Moradie F, et al. Docosahexaenoic acid enrichment in NAFLD is associated with improvements in hepatic metabolism and hepatic insulin sensitivity: a pilot study. European Journal of Clinical Nutrition 2017;71(8):973‐9. [PUBMED: 28294174] - PMC - PubMed
Zhang 2017 {published data only (unpublished sought but not used)}
-
- Zhang Y‐P, Rujuan M, Qing L, Wu T, Ma F. Effects of DHA supplementation on hippocampal volume and cognitive function in older adults with mild cognitive impairment: a 12‐month randomized, double‐blind, placebo‐controlled trial. Journal of Alzheimer's Disease 2016;55(2):497‐507. - PubMed
References to studies excluded from this review
Alekseeva 2000 {published data only}
-
- Alekseeva RI, Sharafetdinov K, Plotnikova OA, Meshcheriakova VA, Mal'tsev GI, Kulakova SN. Effects of a diet including linseed oil on clinical and metabolic parameters in patients with type 2 diabetes mellitus. Voprosy Pitaniia 2000;69(6):32‐5. - PubMed
Baleztena 2015 {published data only}
-
- Baleztena Gurrea J, Ruiz‐Canela M, Pardo M, Castellanos MC, Gozalo MJ, Añorbe T, et al. Utility of heavy food supplement in omega‐3 fatty acids in the prevention of dementia, in relation to the basal nutritional level, in people of advanced age: randomized multicenter study. Revista Española de Geriatría y Gerontología 2015;50:1‐49.
-
- Bes‐Rastrollo M, Baleztena J, Aguirre I, Ruiz‐Canela M. Utility of a rich food supplement omega‐3 fatty acids in the prevention of dementia, in relation to baseline nutritional level, in people with advanced age: multicentre randomised study [Complemento alimenticio rico en ácidos grasos omega‐3 en la prevención de la demencia: estudio aleatorizado multicéntrico]. Nutrición Clínica en Medicina 2015;9(1):38.
Belch 1988 {published data only}
-
- Belch JJ, Ansell D, Madhok R, O'Dowd A, Sturrock RD. Effects of altering dietary essential fatty acids on requirements for non‐steroidal anti‐inflammatory drugs in patients with rheumatoid arthritis: a double blind placebo controlled study. Annals of the Rheumatic Diseases 1988;47(2):96‐104. - PMC - PubMed
Belluzzi 1996 {published and unpublished data}
-
- Belluzzi A, Brignola C, Campieri M, Pera A, Boschi S, Miglioli M. Effect of an enteric‐coated fish‐oil preparation on relapses in Crohn's disease. New England Journal of Medicine 1996;334(24):1557‐60. - PubMed
Berthoux 1992 {published data only}
-
- Berthoux FC, Guerin C, Burgard G, Berthoux P, Alamartine E. One‐year randomized controlled trial with omega‐3 fatty acid‐fish oil in clinical renal transplantation. Transplantation Proceedings 1992;24(6):2578‐82. - PubMed
Borchgrevink 1966 {published and unpublished data}
-
- Borchgrevink CF, Berg KJ, Skaga E, Skjaeggestad O, Stormorken H. Effect of linseed oil on platelet adhesiveness and bleeding time in patients with coronary heart disease. Lancet 1965;ii:980‐2. - PubMed
-
- Borchgrevink CF, Skaga E, Berg KJ, Skjaeggestad O. Absence of prophylactic effect of linolenic acid in patients with coronary heart disease. Lancet 1966;2(456):187‐9. - PubMed
Busnach 1998 {published data only}
-
- Busnach G, Straglioto E, Minetti E, Perego A, Brando B, Broggi ML, et al. Effect of N‐3 polyunsaturated fatty acids on cyclosporine pharmacokinetics in kidney graft recipients: a randomized placebo‐controlled study. Journal of Nephrology 1998;11(2):87‐93. - PubMed
CANN 2015 {unpublished data only}
-
- NCT02525198. The cognitive ageing nutrition and neurogenesis trial (CANN) [A randomised controlled trial in 'at risk' humans investigating the cognitive benefits of a combined flavonoid/fatty acid intervention and underlying mechanisms of action: the Cognitive Aging Nutrition and Neurogenesis 8CANN) trial]. clinicaltrials.gov/ct2/show/NCT02525198 (first received 17 August 2015).
Cappelli 1997 {published data only}
-
- Cappelli P, Di LL, Stuard S, Ballone E, Albertazzi A. N‐3 polyunsaturated fatty acid supplementation in chronic progressive renal disease. Journal of Nephrology 1997;10(3):157‐62. - PubMed
CARES 2015 {published data only}
-
- ISRCTN10431469. Changes in brain function among individuals with a mild memory impairment (CARES). www.isrctn.com/ISRCTN10431469 (first received 21 December 2015). [ISRCTN10431469]
Cheng 1990a {published data only}
-
- Cheng A, Bustami M, Norell MS, Mitchell AG, Ilsey CDJ. The effect of omega‐3 fatty acids on restenosis after coronary angioplasty. European Heart Journal 1990;11:368.
Cheng 1990b {published data only}
-
- Cheng IK, Chan PC, Chan MK. The effect of fish oil dietary supplement on the progression of mesangial IgA glomerulonephritis. Nephrology, Dialysis, Transplantation 1990;5:241‐16. - PubMed
Clark 1993 {published data only}
-
- Clark WF, Parbtani A, Naylor CD, Levinton CM, Muirhead N, Spanner E, et al. Fish oil in lupus nephritis: clinical findings and methodological implications. Kindney International 1993;44(1):75‐86. - PubMed
Clark 1994 {published data only}
-
- Clark WF, Parbtani A. Omega‐3 fatty acid supplementation in clinical and experimental lupus nephritis. American Journal of Kidney Diseases 1994;23(5):644‐7. - PubMed
Clark 2001 {published data only}
-
- Clark WF, Kortas C, Heidenheim AP, Garland J, Spanner E, Parbtani A. Flaxseed in lupus nephritis: a two‐year nonplacebo‐controlled crossover study. Journal of the American College of Nutrition 2001;20(2 Suppl):143‐8. [PUBMED: 11349937] - PubMed
Clausen 1989 {published data only}
-
- Clausen J, Nielsen SA, Kristensen M. Biochemical and clinical effects of an antioxidative supplementation of geriatric patients. A double blind study. Biological Trace Element Research 1989;20(1‐2):135‐51. - PubMed
Diskin 1990 {published data only}
-
- Diskin CJ, Thomas CE, Zellner CP, Lock S, Tanja J. Fish oil to prevent intimal hyperplasia and access thrombosis. Nephron 1990;55(4):445‐7. - PubMed
Donadio 1994 {published data only}
-
- Donadio JJ, Bergstralh EJ, Offord KP, Spencer DC, Holley KE. A controlled trial of fish oil in IgA nephropathy. Mayo Nephrology Collaborative Group. New England Journal of Medicine 1994;331:1194‐9. - PubMed
Doyle 2001 {published data only}
-
- Doyle W, Srivastava A, Crawford MA, Bhatti R, Brooke Z, Costeloe KL. Inter‐pregnancy folate and iron status of women in an inner‐city population. British Journal of Nutrition 2001;86(1):81‐7. - PubMed
Dry 1991 {published and unpublished data}
-
- Dry J, Vincent D. Effect of a fish oil diet on asthma: results of a 1‐year double‐blind study. International Archives of Allergy and Applied Immunology 1991;95(2‐3):156‐7. - PubMed
Ezaki 1999 {published data only}
-
- Ezaki O, Takahashi M, Shigematsu T, Shimamura K, Kimura J, Ezaki H, et al. Long‐term effects of dietary alpha‐linolenic acid from perilla oil on serum fatty acids composition and on the risk factors of coronary heart disease in Japanese elderly subjects. Journal of Nutritional Science and Vitaminology 1999;45(6):759‐72. - PubMed
Feher 2005 {published data only}
-
- Feher J, Kovacs B, Kovacs I, Schveoller M, Papale A, Balacco Gabrieli C. Improvement of visual functions and fundus alterations in early age‐related macular degeneration treated with a combination of acetyl‐L‐carnitine, n‐3 fatty acids, and coenzyme Q10. Ophthalmologica 2005;219(3):154‐66. [PUBMED: 15947501] - PubMed
FISH 2012 {published data only (unpublished sought but not used)}
-
- Browning LM, Walker CG, Mander AP, West AL, Madden J, Gambell JM, et al. Incorporation of eicosapentaenoic and docosahexaenoic acids into lipid pools when given as supplements providing doses equivalent to typical intakes of oily fish. American Journal of Clinical Nutrition 2012;96(4):748‐58. [PUBMED: 22932281] - PMC - PubMed
-
- Walker CG, Browning LM, Stecher L, West AL, Madden J, Jebb SA, et al. Fatty acid profile of plasma NEFA does not reflect adipose tissue fatty acid profile. British Journal of Nutrition 2015;114(5):756‐62. [PUBMED: 26205910] - PubMed
Fonolla 2009 {published data only}
-
- Fonolla J, Lopez‐Huertas E, Machado FJ, Molina D, Alvarez I, Marmol E, et al. Milk enriched with "healthy fatty acids" improves cardiovascular risk markers and nutritional status in human volunteers. Nutrition 2009;25(4):408‐14. [PUBMED: 19084376] - PubMed
Fonolla‐Joya 2016 {published data only}
-
- Fonolla J, Diaz‐Ropero M, Geerlings A, Marti J, Lopez‐Huertas E. Daily intake of a dairy drink enriched with omega‐3 (EPA+DHA) and oleic acid improves cardiovascular markers in healthy postmenopausal women. Atherosclerosis Supplements 2010;11(2):58.
-
- Fonolla J, Diaz‐Ropero P, Marti JL, Rodriguez‐Martinez C, Lopez‐Huertas E. Daily intake of a low lactose dairy drink enriched with omega‐3 (EPA+DHA), oleic acid and calcium improves nutritional and bone status in healthy postmenopausal women. Clinical Nutrition 2011;6(1):86.
-
- Fonolla‐Joya J, Reyes‐García R, García‐Martín A, López‐Huertas E, Muñoz‐Torres M. Daily intake of milk enriched with n‐3 fatty acids, oleic acid, and calcium improves metabolic and bone biomarkers in postmenopausal women. Journal of the American College of Nutrition 2016;35(6):529‐36. [DOI: 10.1080/07315724.2014] - DOI - PubMed
Franzen 1989 {published data only}
-
- Franzen D, Hopp HW, Schanwell M, Hilger HH. Long‐term effect of fish oil and olive oil on plasma lipids in patients with CHD. Zeitschrift fur Kardiologie 1989;78(Suppl 4):28.
Galarraga 2008 {published data only}
Gapparova 2000 {published data only}
-
- Gapparova KM, Pogozheva AV, Kulakova SN, Tutel'ian VA. Effects of omega‐3 polyunsaturated fatty acids of vegetable and animal origin on clinical and metabolic indicators and the intensity of lipid peroxidation in patients with ischemic heart disease and impaired carbohydrate tolerance. Voprosy Pitaniia 2000;69(1‐2):46‐9. - PubMed
Gazso 1992 {published data only}
-
- Gazso A, Horrobin D, Sinzinger H. Influence of omega‐3 fatty acids on the prostaglandin‐metabolism in healthy volunteers and patients suffering from PVD. Agents and Actions. Supplements 1992;37:151‐6. - PubMed
Geusens 1994 {published data only}
-
- Geusens P, Wouters C, Nijs J, Jiang Y, Dequeker J. Long‐term effect of omega‐3 fatty acid supplementation in active rheumatoid arthritis. A 12‐month, double‐blind, controlled study. Arthritis and Rheumatism 1994;37(6):824‐9. - PubMed
Gogos 1998 {published data only}
-
- Gogos CA, Ginopoulos P, Salsa B, Apostolidou E, Zoumbos NC, Kalfarentzos F. Dietary omega‐3 polyunsaturated fatty acids plus vitamin E restore immunodeficiency and prolong survival for severely ill patients with generalized malignancy: a randomized control trial. Cancer 1998;82(2):395‐402. - PubMed
Greatrex 2000 {published data only}
-
- Greatrex JC, Drasdo N, Dresser K. Scotopic sensitivity in dyslexia and requirements for DHA supplementation. Lancet 2000;355(9213):1429‐30. - PubMed
Griffin 1999 {published data only}
-
- Griffin BA, Minihane AM, Furlonger N, Chapman C, Murphy M, Williams D, et al. Inter‐relationships between small, dense low‐density lipoprotein (LDL), plasma triacylglycerol and LDL apoprotein B in an atherogenic lipoprotein phenotype in free‐living subjects. Clinical Science 1999;97(3):269‐76. - PubMed
Hamazaki 1984 {published data only}
-
- Hamazaki T, Tateno S, Shishido H. Eicosapentaenoic acid and IgA nephropathy. Lancet 1984;1(8384):1017‐8. - PubMed
Hansen 1996 {published data only}
-
- Hansen GV, Nielsen L, Kluger E, Thysen M, Emmertsen H, Stengaard PK, et al. Nutritional status of Danish rheumatoid arthritis patients and effects of a diet adjusted in energy intake, fish‐meal, and antioxidants. Scandinavian Journal of Rheumatology 1996;25(5):325‐30. - PubMed
Harris 1991 {published data only}
-
- Harris WS, Windsor SL, Dujovne CA. Effects of four doses of n‐3 fatty acids given to hyperlipidemic patients for six months. Journal of the American College of Nutrition 1991;10(3):220‐7. - PubMed
Hashimoto 2012 {published data only}
-
- Hashimoto M, Yamashita K, Kato S, Tamai T, Matsumoto I, Tanabe Y, et al. Beneficial effects of dietary docosahexaenoic acid intervention on cognitive function in elderly people with very mild dementia in Japan. Alzheimer's and Dementia 2011;1:S610‐1.
-
- Hashimoto M, Yamashita K, Kato S, Tamai T, Tanabe Y, Mitarai M, et al. Beneficial effects of daily dietary omega‐3 polyunsaturated fatty acid supplementation on age related cognitive decline in elderly Japanese with very mild dementia: a 2‐year randomized,double‐blind, placebo‐controlled trial. Journal of Aging Research & Clinical Practice 2012;1(3):193‐201.
Hashimoto 2016 {published data only}
-
- Hashimoto M, Kato S, Tanabe Y, Katakura M, Mamun AA, Ohno M, et al. Beneficial effects of dietary docosahexaenoic acid intervention on cognitive function and mental health of the oldest elderly in Japanese care facilities and nursing homes. Geriatrics & Gerontology International 2017;17(2):330‐7. [PUBMED: 26822516] - PubMed
Hawthorne 1992 {published and unpublished data}
Hogg 1995 {published data only}
-
- Hogg RJ. A randomized, placebo‐controlled, multicenter trial evaluating alternate‐day prednisone and fish oil supplements in young patients with immunoglobulin A nephropathy. American Journal of Kidney Diseases 1995;26(5):792‐6. - PubMed
HOPE epilepsy 2012 {published data only}
-
- NCT01744275. High dose omega 3 fatty acids supplementation in patients with epilepsy: the HOPE‐Epilepsy trial. clinicaltrials.gov/ct2/show/NCT01744275 (first received 6 December 2012).
Huang 1996 {published data only}
-
- Huang YC, Jessup JM, Forse RA, Flickner S, Pleskow D, Anastopoulos HT, et al. N‐3 fatty acids decrease colonic epithelial cell proliferation in high‐risk bowel mucosa. Lipids 1996;31:S313‐7. - PubMed
Huang 2008 {published and unpublished data}
-
- Huang LL, Coleman HR, Kim J, Monasterio F, Wong WT, Schleicher RL, et al. Oral supplementation of lutein/zeaxanthin and omega‐3 long chain polyunsaturated fatty acids in persons aged 60 years or older, with or without AMD. Investigative Ophthalmology & Visual Science 2008;49(9):3864‐9. - PubMed
InTrePad 2013 {published data only}
-
- ACTRN12613000034730. Intervention of testosterone & fish oil as a possible strategy for the prevention of Alzheimer's disease. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=363372 (first received 14 January 2013).
ISRCTN38354847 {published data only}
-
- ISRCTN38354847. A multicentre, double‐blind, randomised, parallel group, placebo‐controlled trial of LAX‐101 (ethyl eicosapentaenoate [EPA]) as adjunct therapy in patients who remain depressed following treatment with standard antidepressant therapy. www.isrctn.com/ISRCTN38354847 (first received 3 February 2003).
Junker 1990 {published data only}
-
- Junker L, Engel S, Berger I, Barleben H. Serum enzymes in grade I hypertensive patients before and following a change in nutrition in relation to polyene acid and electrolyte content. Zeitschrift fur die Gesamte Innere Medizin und Ihre Grenzgebiete 1990;45(11):323‐4. - PubMed
Kachorovskii 1977 {published data only}
-
- Kachorovskii BV, Zhoglo FA, Zaverbnyi MI. Total lipid content in the blood after using fish oil in natural and emulsified forms. Farmatsiia 1977;26(5):86. - PubMed
Kanorskii 2007 {published data only}
-
- Kanorskii SG, Bodrikova VV, Kanorskaia Iu S. Influence of perindopril, rosuvastatin, or omega‐3 polyunsaturated fatty acids on efficacy of antirecurrence therapy with sotalol in patients with persistent atrial fibrillation. Kardiologiia 2007;47(12):39‐44. - PubMed
Karlsson 1998 {published data only}
-
- Karlsson J, Hesselius G, Nygard B, Ronneberg R. Plasma omega‐3 fatty acids before and after nutritional therapy. Journal of Nutritional & Environmental Medicine 1998;8(1):25‐34.
Kaul 1992 {published and unpublished data}
-
- Kaul U, Sanghvi S, Bahl VK, Dev V, Wasir HS. Fish oil supplements for prevention of restenosis after coronary angioplasty. International Journal of Cardiology 1992;35(1):87‐93. - PubMed
Khan 2003 {published and unpublished data}
-
- Khan F, Elherik K, Bolton‐Smith C, Barr R, Hill A, Murrie I, Belch JJF. The effects of dietary fatty acid supplementation on endothelial function and vascular tone in healthy subjects. Cardiovascular Research 2003;59(4):955‐62. - PubMed
Konya 2000 {published data only}
-
- Konya E, Tsuji H, Umekawa T, Kurita T, Iguchi M. Effect of ethyl icosapentate on urinary calcium and oxalate excretion. International Journal of Urology 2000;7(10):361‐5. - PubMed
Kremer 1995 {published data only}
-
- Kremer JM, Lawrence DA, Petrillo GF, Litts LL, Mullaly PM, Rynes RI, et al. Effects of high‐dose fish oil on rheumatoid arthritis after stopping nonsteroidal antiinflammatory drugs. Clinical and immune correlates. Arthritis and Rheumatism 1995;38(8):1107‐14. - PubMed
Kruger 1998 {published data only}
-
- Kruger MC, Coetzer H, Winter R, Gericke G, Papendorp DH. Calcium, gamma‐linolenic acid and eicosapentaenoic acid supplementation in senile osteoporosis. Aging (Milan, Italy) 1998;10(5):385‐94. - PubMed
Kurabayashi 2000 {published data only}
-
- Kurabayashi T, Okada M, Tanaka K. Eicosapentaenoic acid effect on hyperlipidemia in menopausal Japanese women. The Niigata Epadel Study Group. Obstetrics and Gynecology 2000;96(4):521‐8. - PubMed
Lau 1993 {published and unpublished data}
-
- Lau CS, McMahon H, Morley KD, Belch JJF. Effects of Maxepa on non‐steroidal anti‐inflammatory drug useage in patients with mild rheumatoid arthritis. British Journal of Rheumatology 1991;30:137. - PubMed
-
- Lau CS, Morley KD, Belch JJ. Effects of fish oil supplementation on non‐steroidal anti‐inflammatory drug requirement in patients with mild rheumatoid arthritis: a double‐blind placebo controlled study. British Journal of Rheumatology 1993;32(11):982‐9. - PubMed
Leaf 1995 {published data only}
-
- Leaf DA, Connor WE, Barstad L, Sexton G. Incorporation of dietary n‐3 fatty acids into the fatty acids of human adipose tissue and plasma lipid classes. American Journal of Clinical Nutrition 1995;62(1):68‐73. - PubMed
Lee 2010 {published and unpublished data}
-
- Lee TK, Clandinin MT, Hébert M, MacDonald IM. Effect of docosahexaenoic acid supplementation on the macular function of patients with Best vitelliform macular dystrophy: randomized clinical trial. Canadian Journal of Ophthalmology 2010;45(5):514‐9. - PubMed
Leng 1998 {published data only}
-
- Leng GC, Lee AJ, Fowkes FG, Jepson RG, Horrobin D, Lowe GD, et al. Randomised controlled trial of y‐linolenic and eicosapentaenoic acid in peripheral vascular disease. Prostaglandins, Leukotrienes and Essential Fatty Acids 1997;57(2):218.
-
- Leng GC, Lee AJ, Fowkes FG, Jepson RG, Lowe GD, Skinner ER, et al. Randomized controlled trial of gamma‐linolenic acid and eicosapentaenoic acid in peripheral arterial disease. Clinical Nutrition 1998;17(6):265‐71. - PubMed
LipiDiDiet 2016 {published data only}
-
- Freund‐Levi Y, Visser PJ, Kivipelto M, Wieggers RL, Hartmann T, Soininen H. The Lipididiet study: rationale and study design. Alzheimer's & Dementia 2012;1:602.
-
- Soininen H, Visser PJ, Kivipelto M, Hees A, Rouws C, Hartmann T. A clinical trial investigating the effects of Souvenaid in prodromal Alzheimer's disease: progress and baseline characteristics of the Lipididiet study. Neurobiology of Aging 2014;35:S20.
-
- Visser PJ, Soininen H, Kivipelto M, Freund‐Levi Y, Kamphuis P, Wieggers RL, et al. A randomized controlled trial investigating the effects of Souvenaid in prodromal Alzheimer's disease: baseline characteristics of the LipiDiDiet study. Alzheimer's & Dementia 2013;9(4 Suppl):669‐70. [DOI: ]
Loeschke 1996 {published and unpublished data}
-
- Loeschke K, Ueberschaer B, Pietsch A, Gruber E, Ewe K, Wiebecke B, et al. N‐3 fatty acids only delay early relapse of ulcerative colitis in remission. Digestive Diseases and Sciences 1996;41(10):2087‐94. - PubMed
LUTEGA 2013 {published data only}
-
- Arnold C, Winter L, Frohlich K, Jentsch S, Dawczynski J, Jahreis G, et al. Macular xanthophylls and omega‐3 long‐chain polyunsaturated fatty acids in age‐related macular degeneration: a randomized trial. JAMA Ophthalmology 2013;131(5):564‐72. [PUBMED: 23519529] - PubMed
-
- Dawczynski J, Jentsch S, Schweitzer D, Hammer M, Lang GE, Strobel J. Long term effects of lutein, zeaxanthin and omega‐3‐LCPUFAs supplementation on optical density of macular pigment in AMD patients: the LUTEGA study. Graefe's Archive for Clinical and Experimental Ophthalmology 2013;251(12):2711‐23. [PUBMED: 23695657] - PubMed
Lyon Diet Heart 1994 {published data only}
-
- Lorgeril M, Renaud S, Mamelle N, Salen P, Martin JL, Monjaud I, et al. Mediterranean alpha‐linolenic acid‐rich diet in secondary prevention of coronary heart disease. [Erratum appears in Lancet 1995 Mar 18;345(8951):738]. Lancet 1994;343(8911):1454‐9. [DOI: 10.1016/S0140-6736(94)92580-1] - DOI - PubMed
-
- Lorgeril M, Salen P, Caillat‐Vallet E, Hanauer MT, Barthelemy JC, Mamelle N. Control of bias in dietary trial to prevent coronary recurrences: the Lyon Diet Heart Study. European Journal of Clinical Nutrition 1997;51(2):116‐22. - PubMed
-
- Lorgeril M, Salen P, Martin JL, Mamelle N, Monjaud I, Touboul P, et al. Effect of a Mediterranean type of diet on the rate of cardiovascular complications in patients with coronary artery disease. Insights into the cardioprotective effect of certain nutriments. Journal of the American College of Cardiology 1996;28(5):1103‐8. - PubMed
-
- Lorgeril M, Salen P, Martin JL, Monjaud I, Boucher P, Mamelle N. Mediterranean dietary pattern in a randomized trial: prolonged survival and possible reduced cancer rate. Archives of Internal Medicine 1998;158(11):1181‐7. - PubMed
-
- Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation 1999;99(6):779‐85. - PubMed
Maachi 1995 {published data only}
-
- Maachi K, Berthoux P, Burgard G, Alamartine E, Berthoux F. Results of a 1‐year randomized controlled trial with omega‐3 fatty acid fish oil in renal transplantation under triple immunosuppressive therapy. Transplantation Proceedings 1995;27(1):846‐9. - PubMed
Macsai 2008 {published data only}
Mansel 1990 {published data only}
-
- Mansel RE, Harrison BJ, Melhuish J, Sheridan W, Pye JK, Pritchard G, et al. A randomized trial of dietary intervention with essential fatty acids in patients with categorized cysts. Annals of the New York Academy of Sciences 1990;586:288‐94. - PubMed
Mantzaris 1996 {published data only}
-
- Mantzaris GJ, Archavlis E, Zografos C, Petraki K, Spiliades C, Triantafyllou G. A prospective, randomized, placebo‐controlled study of fish oil in ulcerative colitis. Hellenic Journal of Gastroenterology 1996;9(2):138‐41.
Mate‐Jimenez 1991 {published and unpublished data}
-
- Mate J, Castanos R, Garcia‐Semaniego J, Pajares JM. Does dietary fish oil maintain the remission of Crohn's disease: a study case control. Gastroenterology 1991;100:A228.
Matsuyama 2005 {published and unpublished data}
-
- Matsuyama W, Mitsuyama H, Watanabe M, Oonakahara K, Higashimoto I, Osame M, et al. Effects of omega‐3 polyunsaturated fatty acids on inflammatory markers in COPD. Chest 2005;128(6):3817‐27. - PubMed
Middleton 2002 {published data only}
-
- Middleton SJ, Naylor S, Woolner J, Hunter JO. A double‐blind, randomized, placebo‐controlled trial of essential fatty acid supplementation in the maintenance of remission of ulcerative colitis. Alimentary Pharmacology & Therapeutics 2002;16(6):1131‐5. [PUBMED: 12030955] - PubMed
MoodFOOD 2016 {published data only}
NAYAB 2017 {published data only}
NCT01235533 {unpublished data only}
-
- NCT01235533. Fish oil supplementation in late‐life depression. clinicaltrials.gov/ct2/show/NCT01235533 (first received 5 November 2010).
NCT01784042 {published data only}
-
- NCT01784042. Dietary energy restriction and omega‐3 fatty acids on mammary tissue [Effect of dietary energy restriction and omega‐3 fatty acids on mammary tissue and systemic biomarkers of breast cancer risk]. clinicaltrials.gov/ct2/show/NCT01784042 (first received 5 February 2013).
NU‐AGE 2014 {published data only}
-
- Berendsen A, Santoro A, Pini E, Cevenini E, Ostan R, Pietruszka B, et al. Reprint of: a parallel randomized trial on the effect of a healthful diet on inflammageing and its consequences in European elderly people: design of the NU‐AGE dietary intervention study. Mechanisms of Ageing and Development 2014;136:14‐21. - PubMed
-
- Santoro A, Pini E, Scurti M, Palmas G, Berendsen A, Brzozowska A, et al. Combating inflammaging through a Mediterranean whole diet approach: the NU‐AGE project's conceptual framework and design. Mechanisms of Ageing and Development 2014;136‐137:3‐13. - PubMed
NutriMEMO 2014 {published data only}
OFAMS 2012 {published data only}
-
- Torkildsen O, Wergeland S, Bakke S, Beiske AG, Bjerve KS, Hovdal H, et al. Omega‐3 fatty acid treatment in multiple sclerosis (OFAMS Study): a randomized, double‐blind, placebo‐controlled trial. Archives of Neurology 2012;69(8):1044‐51. [PUBMED: 22507886] - PubMed
Okuda 1996 {published data only}
-
- Okuda Y, Mizutani M, Ogawa M, Sone H, Asano M, Asakura Y, et al. Long‐term effects of eicosapentaenoic acid on diabetic peripheral neuropathy and serum lipids in patients with type II diabetes mellitus. Journal of Diabetes and its Complications 1996;10(5):280‐7. - PubMed
OLIVE 1998 {published data only}
-
- Colquhoun DM, Hicks BJ, Somerset S. Rationale and design of the "OLIVE" study (comparison of an olive oil enriched to a low fat diet intervention study using vascular endpoints). Atheroscerosis 1997;134(1‐2):326‐27.
-
- Colquhoun DM, Somerset S, Glasziou PP, Richards D, Weyers J. Comparison of an olive oil enriched diet to a low fat diet intervention study using vascular endpoints: assessed by repeat quantitative angiography (OLIVE study). Australian Journal of Nutrition and Dietetics 1998;55(Suppl 4):S24‐9.
Oslo DIET HEART 1970 {published data only}
-
- Leren P. The Oslo diet‐heart study. Eleven year report. Circulation 1970;42:935‐42. - PubMed
-
- Leren P. The effect of a cholesterol lowering diet in male survivors of myocardial infarction. (A controlled clinical trial). Nordisk Medicin 1967;77(21):658‐61. - PubMed
-
- Leren P. The effect of plasma cholesterol lowering diet in male survivors of myocardial infarction. A controlled clinical trial. Acta Medica Scandinavica 1966;466(Suppl):1‐92. - PubMed
Pogozheva 1997 {published data only}
-
- Pogozheva AV, Rozanova IA, Sorokovoi KV, Karagodina ZV, Levachev MM. Lipid peroxidation in patients with ischemic heart disease, hyperlipidemia and/or hypertension while polyunsaturated omega‐3 fatty acids of plant and animal origin were in their diet. Voprosy Pitaniia 1997;4:32‐5. - PubMed
Pogozheva 2000 {published data only}
-
- Pogozheva AV, Tutel'ian VA, Gapparova KM, Trushina EN, Miagkova MA. The effect of an antiatherosclerotic diet including omega‐3 polyunsaturated fatty acids of marine and plant origins on the indices of cellular and humoral immunity in patients with ischemic heart disease and a disordered carbohydrate tolerance. Voprosy Pitaniia 2000;69(4):33‐5. - PubMed
Puri 2008 {published and unpublished data}
-
- Puri BK, Bydder GM, Manku MS, Clarke A, Waldman AD, Beckmann CF. Reduction in cerebral atrophy associated with ethyl‐eicosapentaenoic acid treatment in patients with Huntington's disease. Journal of International Medical Research 2008;36:896‐905. - PubMed
Quazi 1994 {published data only}
-
- Quazi S, Mohiduzzaman M, Mostafizur RM, Keramat AS. Effect of hilsa (Tenualosa ilisha) fish in hypercholesterolemic subjects. Bangladesh Medical Research Council Bulletin 1994;20(1):1‐7. - PubMed
Sacks 1994 {published data only}
-
- He J, Klag MJ, Appel LJ, Charleston J, Whelton PK. Seven‐year incidence of hypertension in a cohort of middle‐aged African Americans and whites. Hypertension 1998;31(5):1130‐5. - PubMed
-
- Meilahn EN, Kuller LH, Kiss JE, Sacks FM. Coagulation parameters among healthy adults taking fish oil versus placebo. Arteriosclerosis 1990;10:916A.
-
- Sacks FM, Hebert P, Appel LJ, Borhani NO, Applegate WB, Cohen JD, et al. Short report: the effect of fish oil on blood pressure and high‐density lipoprotein‐cholesterol levels in phase I of the Trials of Hypertension Prevention. Journal of Hypertension 1994;12(2):209‐13. - PubMed
-
- Sacks FM, Hebert P, Appel LJ, Borhani NO, Applegate WB, Cohen JD, et al. The effect of fish oil on blood pressure and high‐density lipoprotein‐cholesterol levels in phase I of the trials of hypertension prevention. Journal of Hypertension. Supplement 1994;12(7):S23‐31. - PubMed
-
- Satterfield S, Borhani NO, Whelton P, Goodwin L, Brinkmann C, Charleston J, et al. Recruitment for phase I of the Trials of Hypertension Prevention. American Journal of Preventive Medicine 1993;9(4):237‐43. - PubMed
Saynor 1988 {published data only}
Saynor 1992 {published data only}
-
- Saynor R, Gillott T. Changes in blood lipids and fibrinogen with a note on safety in a long term study on the effects of n‐3 fatty acids in subjects receiving fish oil supplements and followed for seven years. Lipids 1992;27(7):533‐8. - PubMed
Selvais 1995 {published and unpublished data}
-
- Selvais PL, Ketelslegers JM, Buysschaert M, Hermans MP. Plasma endothelin‐1 immunoreactivity is increased following long‐term dietary supplementation with omega‐3 fatty acids in microalbuminuric IDDM patients. Diabetologia 1995;38:253. - PubMed
Shimizu 1995 {published and unpublished data}
-
- Shimizu H, Ohtani K, Tanaka Y, Sato N, Mori M, Shimomura Y. Long‐term effect of eicosapentaenoic acid ethyl (EPA‐E) on albuminuria of non‐insulin dependent diabetic patients. Diabetes Research and Clinical Practice 1995;28(1):35‐40. - PubMed
Singh 1992 {published data only}
-
- Singh RB, Fedacko J, Vargova V, Pella D, Niaz MA, Ghosh S. Effect of low W‐6/W‐3 fatty acid ratio Paleolithic style diet in patients with acute coronary syndromes: a randomized, single blind, controlled trial. World Heart Journal 2012;4(1):71‐84.
Singh 1997 {published and unpublished data}
-
- Singh RB, Niaz MA, Sharma JP, Kumar R, Rastogi V, Moshiri M. Randomized, double‐blind, placebo‐controlled trial of fish oil and mustard oil in patients with suspected acute myocardial infarction: the Indian experiment of infarct survival 4. Cardiovascular Drugs and Therapy 1997;11(3):485‐91. - PubMed
Singh 2002 {published data only}
-
- Pella D, Dubnov G, Singh RB, Sharma R, Berry EM, Manor O. Effects of an Indo‐Mediterranean diet on the omega‐6/omega‐3 ratio in patients at high risk of coronary artery disease: the Indian paradox. World Review of Nutrition and Dietetics 2003;92:74‐80. - PubMed
-
- Singh RB, Dubnov G, Niaz MA, Ghosh S, Singh R, Rastogi SS, et al. Effect of an Indo‐Mediterranean diet on progression of coronary artery disease in high risk patients (Indo‐Mediterranean Diet Heart Study): a randomised single‐blind trial. Lancet 2002;360(9344):1455‐61. - PubMed
Tariq 1989 {published data only}
-
- Tariq T, Close C, Dodds R, Viberti GC, Lee T, Vergani D. The effect of fish‐oil on the remission of type 1 (insulin‐dependent) diabetes in newly diagnosed patients. Diabetologia 1989;32(10):765. - PubMed
Terano 1999 {published and unpublished data}
-
- Terano T, Fujishiro S, Ban T, Yamamoto K, Tanaka T, Noguchi Y, et al. Docosahexaenoic acid supplementation improves the moderately severe dementia from thrombotic cerebrovascular diseases. Lipids 1999;34:S345‐6. - PubMed
Tomer 2001 {published data only}
-
- Tomer A, Kasey S, Connor WE, Clark S, Harker LA, Eckman JR. Reduction of pain episodes and prothrombotic activity in sickle cell disease by dietary n‐3 fatty acids. Thrombosis & Haemostasis 2001;85(6):966‐74. - PubMed
Torjesen 1997 {published data only}
-
- Torjesen PA, Birkeland KI, Anderssen SA, Hjermann I, Holme I, Urdal P. Lifestyle changes may reverse development of the insulin resistance syndrome. The Oslo Diet and Exercise Study: a randomized trial. Diabetes Care 1997;20(1):26‐31. - PubMed
VSDR 2015 {published data only}
-
- Roig‐Revert MJ, Lleo‐Perez A, Zanon‐Moreno V, Vivar‐Llopis B, Marin‐Montiel J, Dolz‐Marco R, et al. Enhanced oxidative stress and other potential biomarkers for retinopathy in type 2 diabetics: beneficial effects of the nutraceutic supplements. BioMed Research International 2015;2015:408180. [DOI: ; PUBMED: 26618168] - PMC - PubMed
Wheaton 2010 {published and unpublished data}
-
- Wheaton DH, Hoffman DR, Locke KG, Watkins RB, Birch DG. Biological safety assessment of docosahexaenoic acid supplementation in a randomized clinical trial for X‐linked retinitis pigmentosa. Archives of Ophthalmology 2010;121(9):1269‐78. - PubMed
Yasui 2001 {published data only}
-
- Yasui T, Tanaka H, Fujita K, Iguchi M, Kohri K. Effects of eicosapentaenoic acid on urinary calcium excretion in calcium stone formers. European Urology 2001;39(5):580‐5. - PubMed
Zinger 1987 {published data only}
-
- Zinger P, Berger I, Liuk K, Taube K, Naumann E. Changes in arterial pressure, serum lipids and thromboxane B2 after using a diet with various levels of eicosapentaenoic acid in patients with hypertension. Klinicheskaia Meditsina 1987;65(1):62‐4. - PubMed
References to studies awaiting assessment
IRCT20100123003140N21 {published data only}
-
- IRCT20100123003140N21. Effect of omega 3 fatty acids supplement on inflammatory factors (IL‐1ß, IL‐6, TNF‐a, IL‐10) and lipid profile in Parkinson disease. en.irct.ir/trial/29861 (added to register 18 April 2017).
References to ongoing studies
AC Omega3 2014 {published data only}
-
- ACTRN12614000732684. The Aboriginal cardiovascular omega‐3 randomised controlled trial [The effect of omega‐3 supplementation on adverse cardiovascular (CV) events among Indigenous Australians with stable coronary artery disease: A randomized controlled trial Query!]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=366337 (first received 10 July 2014).
ACTRN12618000761268 2018 {published data only}
-
- ACTRN12618000761268. A 56 week, double‐blind, randomised study to evaluate the efficacy of testosterone, with and without DHA supplementation on cerebral amyloid load in known brain amyloid‐PET positive men with subjective memory complaints. www.anzctr.org.au/ACTRN12618000761268.aspx (added to register 7 May 2018).
AFORRD 2010 {published and unpublished data}
-
- Farmer AJ, Oke J, Hardeman W, Tucker L, Sutton S, Kinmonth A‐L, et al. The effect of a brief action planning intervention on adherence to double‐blind study medication, compared to a standard trial protocol, in the Atorvastatin in Factorial with Omega EE90 Risk Reduction in Diabetes (AFORRD) clinical trial: a cluster randomised sub‐study. Diabetes Research and Clinical Practice 2016;120:56‐64. [DOI: 10.1016/j.diabres.2016.07.004] - DOI - PMC - PubMed
-
- Holman RR, Paul S, Farmer A, Tucker L, Stratton IM, Neil HA, et al. Atorvastatin in factorial with omega‐3 EE90 risk reduction in diabetes (AFORRD): a randomised controlled trial. Diabetologia 2009;52(1):50‐9. - PubMed
-
- Neil HA, Ceglarek U, Thiery J, Paul S, Farmer A, Holman RR. Impact of atorvastatin and omega‐3 ethyl esters 90 on plasma plant sterol concentrations and cholesterol synthesis in type 2 diabetes: a randomised placebo controlled factorial trial. Atherosclerosis 2010;213(2):512‐7. - PubMed
Bartold 2010 {published data only}
-
- ACTRN12610000594022. Fish oil as adjunct therapy for periodontitis [Clinical efficacy of fish oil as adjunct therapy for patients with chronic periodontitis]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=335470 (first received 23 July 2010).
-
- Park B. Impact of omega‐3 fatty acids on periodontal inflammation (PhD thesis). University of Adelaide digital.library.adelaide.edu.au/dspace/bitstream/2440/92351/3/02whole.pdf 2015.
Beyond Aging Project 2015 {published data only}
-
- ACTRN12610000032055. The Beyond Ageing Project: a selective prevention trial using novel pharmacotherapies in an older age cohort at risk for depression Query! [In older adults (60+ years) at risk for depression, can sertraline and/or omega‐3 fatty acids compared with a placebo, reduce or prevent depressive symptoms, incidence of new cases of depression and/or cognitive decline]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=308413 (first received 22 October 2009).
-
- Cockayne NL, Duffy SL, Bonomally R, English A, Amminger PG, Mackinnon A, et al. The Beyond Ageing Project Phase 2: a double‐blind, selective prevention, randomised, placebo‐controlled trial of omega‐3 fatty acids and sertraline in an older age cohort at risk for depression: study protocol for a randomized controlled trial. Trials 2015;16:247. - PMC - PubMed
Chandrakala 2010 {published data only (unpublished sought but not used)}
-
- Chandrakala G, Arpana G, Rao PV. Long‐term effects of a reduced fat diet intervention in pre‐diabetes. 70th Scientific Sessions of the American Diabetes Association; 25‐29 June 2010; Orlando (FL). 2010; Vol. professional.diabetes.org/meeting/scientific‐sessions/70th‐scientific‐se....
-
- Chandrakala G, Arpana G, Sreenivas T, Rao PV. Low‐fat (< 20%) diets prevent type 2 diabetes mellitus. Diabetes 2012;61:A190.
ChiCTR‐TRC‐12002014 {published data only}
-
- ChiCTR‐TRC‐12002014. Influence of different source of n‐3 fatty acid on plasma lipid in moderately hypercholesterolemia subject and the valid dosage. www.chictr.org.cn/hvshowproject.aspx?id=2374 (first received 3 May 2015).
DO HEALTH {published data only}
-
- Do‐Health. DO‐HEALTH trial web site. do‐health.eu/wordpress (accessed prior to 10 May 2018).
-
- NCT01745263. DO‐HEALTH / vitamin D3 ‐ omega3 ‐ home exercise ‐ healthy ageing and longevity trial (DO‐HEALTH). clinicaltrials.gov/ct2/show/NCT01745263 (first received 10 December 2012).
EVAPORATE 2016 {published data only}
-
- NCT02926027. Effect of Vascepa on improving coronary atherosclerosis in people with high triglycerides taking statin therapy (EVAPORATE). clinicaltrials.gov/ct2/show/NCT02926027 (first posted 6 October 2016).
LO‐MAPT 2018 {published data only}
-
- NCT03691519. Prevention of cognitive decline in older adults with low DHA/EPA index in red blood cells (LO‐MAPT). clinicaltrials.gov/show/NCT03691519 (added to registry 1 October 2018).
MAPT PLUS {published data only}
-
- NCT01513252. Long‐term effects of interventional strategies to prevent cognitive decline in elderly (MAPT‐PLUS). clinicaltrials.gov/ct2/show/NCT01513252 (first received 20 January 2012).
MTG 2018 {published data only}
-
- NCT03448185. Improving betabolic health in patients with diastolic dysfunction (MTG). clinicaltrials.gov/ct2/show/NCT03448185 (first posted 28 February 2018).
NCT00010868 {published data only}
-
- NCT00010868. Omega‐3 fatty acids in bipolar disorder [Omega‐3 fatty acids in bipolar disorder prophylaxis]. clinicaltrials.gov/ct2/show/NCT00010868 (first received 5 February 2001).
NCT00309439 {published data only}
-
- NCT00309439. ALA and prostate cancer [Studies of serum PSA to help resolve the current implication of alpha‐linolenic acid (ALA) and prostate cancer]. clinicaltrials.gov/ct2/show/NCT00309439 (first received 31 March 2006).
NCT02211560 {published data only}
-
- NCT02211560. Investigating a phosphatidylserine based dietary approach for the management of mild cognitive impairment [A, multi‐center, double‐blind, randomized, placebo‐controlled study for the efficacy of phosphatidylserine in mild cognitive impairment (MCI)]. clinicaltrials.gov/ct2/show/NCT02211560 (first received 7 August 2014).
NCT02295059 {published data only}
-
- NCT02295059. Omega 3 fatty acids and ERPR(‐)HER2(+/‐) breast cancer prevention [Omega‐3 fatty acids and ERPR(‐) and HER‐2/Neu(+/‐) breast cancer prevention]. clinicaltrials.gov/ct2/show/NCT02295059 (first received 20 November 2014).
NCT02719327 {published data only}
-
- NCT02719327. Brain amyloid and vascular effects of eicosapentaenoic acid (BRAVE‐EPA) [Impact of icosapent ethyl on Alzheimers disease biomarkers in preclinical adults]. clinicaltrials.gov/ct2/show/NCT02719327 (first received 25 March 2016).
NCT03784963 {published data only}
-
- NCT03784963. Efficacy of omega‐3 fatty acid therapy in preventing gastrointestinal bleeding in patients with CF‐LVAD. clinicaltrials.gov/ct2/show/NCT03784963 (first posted 24 December 2018).
NCT03806426 {published data only}
-
- NCT03806426. Effect of EPA‐FFA on polypectomy in familial adenomatous polyposis. clinicaltrials.gov/show/NCT03806426 (first received January 2019).
OMEMI 2014 {published data only}
-
- Laake K, Seljeflot I, Schmidt EB, Myhre P, Tveit A, Arnesen H, et al. Serum fatty acids, traditional risk factors, and comorbidity as related to myocardial injury in an elderly population with acute myocardial infarction. Journal of Lipids 2016;2016:4945720. [dx.doi.org/10.1155/2016/4945720] - PMC - PubMed
-
- NCT01841944. Omega‐3 fatty acids in elderly patients with acute myocardial infarction (OMEMI) [Giving omega‐3 fatty acids to elderly patients diagnosed with acute myocardial infarction to investigate the effect on cardiovascular morbidity and mortality]. clinicaltrials.gov/ct2/show/NCT01841944 (first received 29 April 2013).
POSEIDON 2018 {published data only}
-
- NCT03406897. Pilot study of omega‐3 and vitamin D in high‐dose in type I diabetic patients (POSEIDON). clinicaltrials.gov/show/NCT03406897 (added to register 28 January 2018).
Shinto 2015 {published data only}
-
- Bowman LG, Silbert CL, Dodge HH, Lahna D, Hagen K, Murchison FC, et al. Randomized trial of marine n‐3 polyunsaturated fatty acids for the prevention of cerebral small vessel disease and inflammation in aging (PUFA Trial): rationale, design and baseline results. Nutrients 2019;11(4):735. [DOI: 10.3390/nu11040735] - DOI - PMC - PubMed
-
- NCT01953705. n‐3 PUFA for vascular cognitive aging [Omega 3 PUFA for the vascular component of age‐related cognitive decline]. clinicaltrials.gov/ct2/show/NCT01953705 (first received 1 October 2013).
-
- Shinto L, Silbert LC, Dodge HH, Quinn JF, Howieson D, Kaye J, et al. Omega 3 fatty acids for the prevention of vascular cognitive aging: methods and rationale for a phase II trial. Alzheimer's & Dementia 2015;11(7):610.
STRENGTH 2015 {published data only}
-
- EudraCT 2014‐001069‐28. A long‐term outcomes study to assess statin residual risk reduction with EpaNova in high cardiovascular risk patients with hypertriglyceridemia (STRENGTH). www.clinicaltrialsregister.eu/ctr‐search/search?query=2014‐001069‐28 (first received 10 February 2015).
SUPERIORSVG 2010 {published data only}
-
- Deb S, Singh SK, Souza D, Chu MW, Whitlock R, Meyer SR, on behalf of The, Superior SVG Study Investigators. SUPERIOR SVG: no touch saphenous harvesting to improve patency following coronary bypass grafting (a multi‐centre randomized control trial, NCT01047449). Journal of Cardiothoracic Surgery 2019;14:85. [DOI: 10.1186/s13019-019-0887-x] - DOI - PMC - PubMed
-
- NCT01047449. Improving the results of heart bypass surgery using new approaches to surgery and medication (SUPERIORSVG) [Surgical and pharmacological novel interventions to improve overall results of saphenous vein graft patency in coronary artery bypass grafting surgery: an international multi‐center randomized controlled clinical trial]. clinicaltrials.gov/ct2/show/NCT01047449 (first received 13 January 2010).
UMIN000012825 {published data only}
-
- UMIN000012825. Effect of polyunsaturated fatty acids on vascular healing process in hyper‐cholesterolemic patients with acute coronary syndrome. upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000014981 (first received 1 February 2015).
Additional references
Abdelhamid 2018b
Abdelhamid 2019
-
- Abdelhamid A, Hooper L, Sivakaran R, Hayhoe RP, Welch A. The relationship between omega 3, omega 6 and total polyunsaturated fat and musculoskeletal health and functional status in adults: a systematic review and meta analysis of RCTs. Calcified Tissue International 2019;105:353‐72. [DOI: ] - PubMed
AHA 2016
-
- American Heart Association. Fish and Omega‐3 Fatty Acids. www.heart.org/HEARTORG/HealthyLiving/HealthyEating/HealthyDietGoals/Fish... (accessed 24 November 2017).
Aung 2018
-
- Aung T, Halsey J, Kromhout D, Gerstein HC, Marchioli R, Tavazzi L, et al. Associations of omega‐3 fatty acid supplement use with cardiovascular disease risks: meta‐analysis of 10 trials involving 77 917 individuals. JAMA Cardiology 2018;3(3):225‐34. [DOI: 10.1001/jamacardio.2017.5205] - DOI - PMC - PubMed
Balk 2016
-
- Balk EM, Adam GP, Langberg V, Halladay C, Chung M, Lin L, et al. Omega‐3 fatty acids and cardiovascular disease: an updated systematic review. Rockville (MD): Agency for Healthcare Research and Quality; 2016. Evidence report/technology assessment no. 223. AHRQ Publication No. 16‐E002‐EF. www.effectivehealthcare.ahrq.gov/reports/final.cfm. [DOI: 10.23970/AHRQEPCERTA223] - DOI - PubMed
Ballard‐Barbash 1987
-
- Ballard‐Barbash R, Callaway CW. Marine fish oils: role in prevention of coronary artery disease. Mayo Clinic Proceedings 1987;62:113‐8. - PubMed
Bang 1972
-
- Bang HO, Dyerberg J. Plasma lipids and lipoproteins in Greenlandic West Coast Eskimos. Acta Medica Scandinavica 1972;192:85‐94. - PubMed
Bang 1976
-
- Bang HO, Dyerberg J, Hjorne N. The composition of food consumed by Greenland Eskimos. Acta Medica Scandinavica 1976;200:69‐73. - PubMed
Berkley 1995
-
- Berkley CS, Hoaglin DC, Mosteller F, Colditz GA. A random‐effects regression model for meta‐analysis. Statistics in Medicine 1995;14:395‐411. - PubMed
Bhatnagar 2003
-
- Bhatnagar D, Durrington PN. Omega‐3 fatty acids: their role in the prevention and treatment of atherosclerosis related risk factors and complications. International Journal of Clinical Practice 2003;57(4):305‐14. - PubMed
BMJ 2005
BNF 1999
-
- British Nutrition Foundation. N‐3 fatty acids and health. London: British Nutrition Foundation; 1999. Briefing paper.
Bourdon 2010
-
- Bourdon JA, Bazinet TM, Arnason TT, Kimpe LE, Blais JM, White PA. Polychlorinated biphenyls (PCBs) contamination and aryl hydrocarbon receptor (AhR) agonist activity of omega‐3 polyunsaturated fatty acid supplements: implications for daily intake of dioxins and PCBs. Food and Chemical Toxicology 2010;48(11):3093‐7. - PubMed
Brainard 2019
-
- Brainard J, Jimoh OF, Deane K, Biswas P, Donaldson D, Maas K, et al. Omega‐3, omega‐6 and total polyunsaturated fat for cognition and dementia: systematic review and meta‐analysis of RCTs. submitted 2019.
Brown 2019
Brunner 2009
Burr 1993
-
- Burr ML. Fish and ischaemic heart disease. World Review of Nutrition and Dietetics 1993;72:49‐60. - PubMed
Cade 2007
Calabresi 2004
-
- Calabresi L, Villa B, Canavesi M, Sirtori CR, James RW, Bernini F, et al. An omega‐3 polyunsaturated fatty acid concentrate increases plasma high‐density lipoprotein 2 cholesterol and paraoxonase levels in patients with familial combined hyperlipidemia. Metabolism 2004;53(2):153‐8. - PubMed
Campbell 2013
Chang 2013
Chowdhury 2012
Deane 2019
-
- Deane KH, Jimoh OF, Biswas P, O'Brien AT, Hanson S, Abdelhamid AS, et al. Omega‐3 and polyunsaturated fat for prevention of depression and anxiety symptoms: a systematic review and meta‐analysis of randomised trials. British Journal of Psychiatry 2019;online:1‐8. [DOI: 10.1192/bjp.2019.234] - DOI - PubMed
Delgado‐Lista 2012
-
- Delgado‐Lista J, Perez‐Martinez P, Lopez‐Miranda J, Perez‐Jimenez F. Long chain omega‐3 fatty acids and cardiovascular disease: a systematic review. British Journal of Nutrition 2012;107(Suppl 2):S201‐13. - PubMed
Egger 1997
Engell 2013
-
- Engell RE, Sanman E, Lim SS, Mozaffarian D. Seafood omega‐3 intake and risk of coronary heart disease death: an updated meta‐analysis with implications for attributable burden. Lancet 2013;381(Special Issue):S45. [DOI: 10.1016/S0140-6736(13)61299-4] - DOI
FSA 2000
-
- Food Standards Agency UK. Dioxins and PCBs in the UK diet: 1997 total diet study samples. Food Surveillance Information Sheet. Number 4/00; 2000. [FSIS 4/00]
Geelen 2004
-
- Geelen A, Brouwer IA, Zock PL, Katan MB. Antiarrhythmic effects of n‐3 fatty acids: evidence from human studies. Current Opinion in Lipidology 2004;15:25‐30. - PubMed
GRADE Working Group 2004
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime, Inc). GRADEpro GDT. Version accessed 30 September 2017. Hamilton (ON): McMaster University (developed by Evidence Prime, Inc), 2015.
Hanson 2019
Hauck 1991
-
- Hauck WW, Gilliss CL, Donner A, Gortner S. Randomisation by cluster. Nursing Research 1991;40(6):356‐8. - PubMed
He 2013
-
- He Z, Yang L, Tian J, Yang K, Wu J, Yao Y. Efficacy and safety of omega‐3 fatty acids for the prevention of atrial fibrillation: a meta‐analysis. Canadian Journal of Cardiology 2013;29(2):196‐203. - PubMed
Higgins 2003
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Hooper 2015
Hooper 2018
Hooper 2019
-
- Hooper L, Abdelhamid AS, Brainard J, Deane KH, Song F, the PUFAH Group. Creation of a database to assess effects of omega‐3, omega‐6 and total polyunsaturated fats on health: methodology for a set of systematic reviews. BMJ Open 2019;9:e029554. [DOI: 10.1136/bmjopen-2019-029554] - DOI - PMC - PubMed
Horton 2005
Hu 2019
JECFA 2001
-
- Joint FAO/WHO Expert Committee on Food Additives. Summary of the fifty‐seventh meeting of JEFCA, 2001. apps.who.int/iris/bitstream/handle/10665/42578/WHO_TRS_909.pdf?sequence=1.
Khoueiry 2013
-
- Khoueiry G, Abi Rafeh N, Sullivan E, Saiful F, Jaffery Z, Kenigsberg DN, et al. Do omega‐3 polyunsaturated fatty acids reduce risk of sudden cardiac death and ventricular arrhythmias? A meta‐analysis of randomized trials. Heart & Lung 2013;42(4):251‐6. - PubMed
Kotwal 2012
-
- Kotwal S, Jun M, Sullivan D, Perkovic V, Neal B. Omega 3 fatty acids and cardiovascular outcomes: systematic review and meta‐analysis. Circulation. Cardiovascular Quality and Outcomes 2012;5(6):808‐18. - PubMed
Kris‐Etherton 2002
-
- Kris‐Etherton PM, Harris WS, Appel LJ, for the Nutrition Committee of the American Heart Association. Fish consumption, fish oil, omega 3 fatty acids, and cardiovascular disease. Circulation 2002;106:2747‐57. - PubMed
Kwak 2012
-
- Kwak SM, Myung SK, Lee YJ, Seo HG. Efficacy of omega‐3 fatty acid supplements (eicosapentaenoic acid and docosahexaenoic acid) in the secondary prevention of cardiovascular disease: a meta‐analysis of randomized, double‐blind, placebo‐controlled trials. Archives of Internal Medicine 2012;172(9):686‐94. - PubMed
Larsson 2012
-
- Larsson SC, Orsini N, Wolk A. Long‐chain omega‐3 polyunsaturated fatty acids and risk of stroke: a meta‐analysis. European Journal of Epidemiology 2012;27(12):895‐901. - PubMed
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Levine 2005
Li 1999
-
- Li D, Sinclair A, Wilson A, Nakkote S, Kelly F, Abedin L, et al. Effect of dietary alpha‐linolenic acid on thrombotic risk factors in vegetarian men. American Journal of Clinical Nutrition 1999;69:872‐82. - PubMed
Liem 1997
-
- Liem AK, Theelen RM. Dioxins: Chemical Analysis, Exposure and Risk Assessment. Utrecht: Universiteit Utrecht, 1997. [ISBN 90 393 2012 8]
MAFF 1998A
-
- MAFF UK. Concentrations of metals and other elements in marine fish and shellfish. Food Surveillance Information Sheet no.151; 1998. [FSIS 151]
Mariani 2013
Mortensen 2019
Nettleton 1991
-
- Nettleton JA. Omega‐3 fatty acids: comparison of plant and seafood sources in human nutrition. Journal of the American Dietetic Association 1991;91:331‐7. - PubMed
NICE 2016
-
- NICE. Cardiovascular disease: risk assessment and reduction, including lipid modification clinical guideline [CG181]. www.nice.org.uk/guidance/cg181 July 2014, last updated September 2016.
Page 2019
-
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. Draft version (29th January 2019). In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editor(s). Cochrane Handbook for Systematic Reviews of Interventions. London: Cochrane, 2019.
Papanikolaou 2014
Pawlosky 2001
-
- Pawlosky RJ, Hibbeln JR, Novotny JA, Salem N Jr. Physiological compartmental analysis of alpha‐linolenic acid metabolism in adult humans. Journal of Lipid Research 2001;42:1257‐65. - PubMed
Rizos 2012
-
- Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega‐3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta‐analysis. JAMA 2012;308(10):1024‐33. - PubMed
SACN COT 2004
-
- Scientific Advisory Committee on Nutrition, Committee on Toxicity. Advice on fish consumption: benefits & risks. London, United Kingdom: Her Majesty's Stationary Office, 2004.
Savovic 2012
-
- Savovic J, Jones H, Altman D, Harris R, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta‐epidemiological studies. Health Technology Assessment 2012;16:1‐82. - PubMed
Scandinavian Simvastatin Survival Study Group 1994
-
- Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344(8934):1383‐9. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. - PubMed
Schünemann 2017
-
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Akl E, et al. on behalf of the Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Sethi 2016
-
- Sethi A, Bajaj A, Khosla S, Arora RR. Statin use mitigate the benefit of omega‐3 fatty acids supplementation: a meta‐regression of randomized trials. American Journal of Therapeutics 2016;23(3):e737‐48. - PubMed
Sharp 1998
-
- Sharp S. Meta‐analysis regression. Stata Technical Bulletin 1998;42:16‐22.
Simopoulos 1992
-
- Simopoulos AP, Norman HA, Gillaspy JE, Duke JA. Common purslane: a source of omega‐3 fatty acids and antioxidants. Journal of the American College of Nutrition 1992;11(4):374‐82. - PubMed
Skulas‐Ray 2019
Stark 2016
Thorpe 2017
-
- Thorpe G, Ajabnoor S, Ahmed Z, Abdelhamid A, Hooper L. Dietary polyunsaturated fat for prevention and treatment of inflammatory bowel disease. PROSPERO 2017;www.crd.york.ac.uk/prospero/display_record.php?RecordID=68704:CRD4201706....
Trikalinos 2012
Turgeon 2015
USFDA 1995
-
- US Food, Drug Administration. What you need to know about mercury in fish and shellfish. www.fda.gov/food/foodborneillnesscontaminants/metals/ucm351781.htm (accessed May 2018).
USFDA 2000
-
- US Food, Drug Administration. Center for Food Safety and Applied Nutrition, Office of Nutritional Products. Labeling, and dietary supplements: letter regarding dietary supplement health claim for omega‐3 fatty acids and coronary heart disease (docket No. 91N‐0103). www.fda.gov/Food/LabelingNutrition/ucm073992.htm#cardio 2004.
White 2005
WHO 2017
-
- World Health Organization. Cardiovascular Diseases (CVDs). Fact sheets; May 2017. www.who.int/mediacentre/factsheets/fs317/en (accessed 24 November 2017).
Wood 2008
World Bank 2018
-
- World Bank. World Bank Country and Lending Groups ‐ country classification. datahelpdesk.worldbank.org/knowledgebase/articles/906519‐world‐bank‐coun... (accessed 3 April 2018).
Zhao 2009
-
- Zhao YT, Chen Q, Sun YX, Li XB, Zhang P, Xu Y, et al. Prevention of sudden cardiac death with omega‐3 fatty acids in patients with coronary heart disease: a meta‐analysis of randomized controlled trials. Annals of Medicine 2009;41(4):301‐10. - PubMed
Zheng 2014
-
- Zheng T, Zhao J, Wang Y, Liu W, Wang Z, Shang Y, et al. The limited effect of omega‐3 polyunsaturated fatty acids on cardiovascular risk in patients with impaired glucose metabolism: a meta‐analysis. Clinical Biochemistry 2014;47(6):369‐77. - PubMed
References to other published versions of this review
Abdelhamid 2018a
Hooper 2004
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

