Identification of microRNAs in developing wheat grain that are potentially involved in regulating grain characteristics and the response to nitrogen levels
- PMID: 32103721
- PMCID: PMC7045451
- DOI: 10.1186/s12870-020-2296-7
Identification of microRNAs in developing wheat grain that are potentially involved in regulating grain characteristics and the response to nitrogen levels
Abstract
Background: MicroRNAs (miRNAs) play crucial roles in the regulation of plant development and growth, but little information is available concerning their roles during grain development under different nitrogen (N) application levels. Our objective was to identify miRNAs related to the regulation of grain characteristics and the response to different N fertilizer conditions.
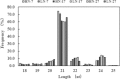
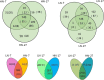
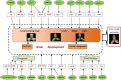
Results: A total of 79 miRNAs (46 known and 33 novel miRNAs) were identified that showed significant differential expression during grain development under both high nitrogen (HN) and low nitrogen (LN) treatments. The miRNAs that were significantly upregulated early in grain development target genes involved mainly in cell differentiation, auxin-activated signaling, and transcription, which may be associated with grain size; miRNAs abundant in the middle and later stages target genes mainly involved in carbohydrate and nitrogen metabolism, transport, and kinase activity and may be associated with grain filling. Additionally, we identified 50 miRNAs (22 known and 28 novel miRNAs), of which 11, 9, and 39 were differentially expressed between the HN and LN libraries at 7, 17, and 27 days after anthesis (DAA). The miRNAs that were differentially expressed in response to nitrogen conditions target genes involved mainly in carbohydrate and nitrogen metabolism, the defense response, and transport as well as genes that encode ubiquitin ligase. Only one novel miRNA (PC-5p-2614_215) was significantly upregulated in response to LN treatment at all three stages, and 21 miRNAs showed significant differential expression between HN and LN conditions only at 27 DAA. We therefore propose a model for target gene regulation by miRNAs during grain development with N-responsive patterns.
Conclusions: The potential targets of the identified miRNAs are related to various biological processes, such as carbohydrate/nitrogen metabolism, transcription, cellular differentiation, transport, and defense. Our results indicate that miRNA-mediated networks, via posttranscriptional regulation, play crucial roles in grain development and the N response, which determine wheat grain weight and quality. Our study provides useful information for future research of regulatory mechanisms that focus on improving grain yield and quality.
Keywords: Differentially expressed miRNAs; Grain development; Nitrogen application level; Wheat.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Development-associated microRNAs in grains of wheat (Triticum aestivum L.).BMC Plant Biol. 2013 Sep 23;13:140. doi: 10.1186/1471-2229-13-140. BMC Plant Biol. 2013. PMID: 24060047 Free PMC article.
-
Screening of differentially expressed microRNAs and target genes in two potato varieties under nitrogen stress.BMC Plant Biol. 2022 Oct 8;22(1):478. doi: 10.1186/s12870-022-03866-5. BMC Plant Biol. 2022. PMID: 36207676 Free PMC article.
-
Small RNA and Degradome Sequencing Reveal Complex Roles of miRNAs and Their Targets in Developing Wheat Grains.PLoS One. 2015 Oct 1;10(10):e0139658. doi: 10.1371/journal.pone.0139658. eCollection 2015. PLoS One. 2015. PMID: 26426440 Free PMC article.
-
Genetic regulation of the traits contributing to wheat nitrogen use efficiency.Plant Sci. 2021 Feb;303:110759. doi: 10.1016/j.plantsci.2020.110759. Epub 2020 Nov 15. Plant Sci. 2021. PMID: 33487345 Review.
-
miRNAomic Approach to Plant Nitrogen Starvation.Int J Genomics. 2021 Nov 9;2021:8560323. doi: 10.1155/2021/8560323. eCollection 2021. Int J Genomics. 2021. PMID: 34796230 Free PMC article. Review.
Cited by
-
MiR396 regulatory network and its expression during grain development in wheat.Protoplasma. 2021 Jan;258(1):103-113. doi: 10.1007/s00709-020-01556-3. Epub 2020 Sep 14. Protoplasma. 2021. PMID: 32929630
-
Potassium Deficiency Significantly Affected Plant Growth and Development as Well as microRNA-Mediated Mechanism in Wheat (Triticum aestivum L.).Front Plant Sci. 2020 Aug 14;11:1219. doi: 10.3389/fpls.2020.01219. eCollection 2020. Front Plant Sci. 2020. PMID: 32922417 Free PMC article.
-
Development and characterization of nitrogen and phosphorus use efficiency responsive genic and miRNA derived SSR markers in wheat.Heredity (Edinb). 2022 Jun;128(6):391-401. doi: 10.1038/s41437-022-00506-4. Epub 2022 Feb 7. Heredity (Edinb). 2022. PMID: 35132208 Free PMC article.
-
Transcriptome Analysis Provides Insights into Grain Filling in Foxtail Millet (Setaria italica L.).Int J Mol Sci. 2020 Jul 16;21(14):5031. doi: 10.3390/ijms21145031. Int J Mol Sci. 2020. PMID: 32708737 Free PMC article.
-
Integrated microRNA and transcriptome profiling reveal key miRNA-mRNA interaction pairs associated with seed development in Tartary buckwheat (Fagopyrum tataricum).BMC Plant Biol. 2021 Mar 9;21(1):132. doi: 10.1186/s12870-021-02914-w. BMC Plant Biol. 2021. PMID: 33750309 Free PMC article.
References
-
- Braun HJ, Atlin G, Payne T. Muti-location testing as a tool to identify plant response to global climate change. In: Reynolds MP, editor. Climate change and crop production. Wallingford: CABI; 2010. pp. 115–138.
-
- Cakir C, Gillespie ME, Scofield SR. Rapid determination of gene function by vius-induced gene silencing in wheat and barley. Crop Sci. 2010;50:S77–S84. doi: 10.2135/cropsci2009.10.0567. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

