Permanent Pro-Tumorigenic Shift in Adipose Tissue-Derived Mesenchymal Stromal Cells Induced by Breast Malignancy
- PMID: 32093026
- PMCID: PMC7072834
- DOI: 10.3390/cells9020480
Permanent Pro-Tumorigenic Shift in Adipose Tissue-Derived Mesenchymal Stromal Cells Induced by Breast Malignancy
Abstract
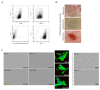
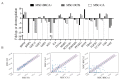
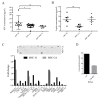
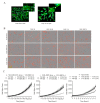
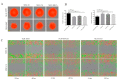
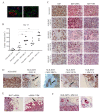
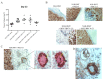
During cancer progression, breast tumor cells interact with adjacent adipose tissue, which has been shown to be engaged in cancer aggressiveness. However, the tumor-directed changes in adipose tissue-resident stromal cells affected by the tumor-stroma communication are still poorly understood. The acquired changes might remain in the tissue even after tumor removal and may contribute to tumor relapse. We investigated functional properties (migratory capacity, expression and secretion profile) of mesenchymal stromal cells isolated from healthy (n = 9) and tumor-distant breast adipose tissue (n = 32). Cancer patient-derived mesenchymal stromal cells (MSCs) (MSC-CA) exhibited a significantly disarranged secretion profile and proliferation potential. Co-culture with MDA-MB-231, T47D and JIMT-1, representing different subtypes of breast cancer, was used to analyze the effect of MSCs on proliferation, invasion and tumorigenicity. The MSC-CA enhanced tumorigenicity and altered xenograft composition in immunodeficient mice. Histological analysis revealed collective cell invasion with a specific invasive front of EMT-positive tumor cells as well as invasion of cancer cells to the nerve-surrounding space. This study identifies that adipose tissue-derived mesenchymal stromal cells are primed and permanently altered by tumor presence in breast tissue and have the potential to increase tumor cell invasive ability through the activation of epithelial-to-mesenchymal transition in tumor cells.
Keywords: adipose tissue; breast cancer; mesenchymal stromal cells; perineural invasion; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
LincK contributes to breast tumorigenesis by promoting proliferation and epithelial-to-mesenchymal transition.J Hematol Oncol. 2019 Feb 22;12(1):19. doi: 10.1186/s13045-019-0707-8. J Hematol Oncol. 2019. PMID: 30795783 Free PMC article.
-
Enhanced metastatic capacity of breast cancer cells after interaction and hybrid formation with mesenchymal stroma/stem cells (MSC).Cell Commun Signal. 2018 Jan 5;16(1):2. doi: 10.1186/s12964-018-0215-4. Cell Commun Signal. 2018. PMID: 29329589 Free PMC article.
-
Altered features and increased chemosensitivity of human breast cancer cells mediated by adipose tissue-derived mesenchymal stromal cells.BMC Cancer. 2013 Nov 9;13:535. doi: 10.1186/1471-2407-13-535. BMC Cancer. 2013. PMID: 24209831 Free PMC article.
-
Mesenchymal stem cell secretome and regenerative therapy after cancer.Biochimie. 2013 Dec;95(12):2235-45. doi: 10.1016/j.biochi.2013.05.010. Epub 2013 Jun 5. Biochimie. 2013. PMID: 23747841 Free PMC article. Review.
-
Roles of cell fusion between mesenchymal stromal/stem cells and malignant cells in tumor growth and metastasis.FEBS J. 2021 Mar;288(5):1447-1456. doi: 10.1111/febs.15483. Epub 2020 Jul 24. FEBS J. 2021. PMID: 33070450 Review.
Cited by
-
Upregulation of CPNE7 in mesenchymal stromal cells promotes oral squamous cell carcinoma metastasis through the NF-κB pathway.Cell Death Discov. 2021 Oct 14;7(1):294. doi: 10.1038/s41420-021-00684-w. Cell Death Discov. 2021. PMID: 34650058 Free PMC article.
-
Adipose tissue-derived stem cells in breast reconstruction: a brief review on biology and translation.Stem Cell Res Ther. 2021 Jan 6;12(1):8. doi: 10.1186/s13287-020-01955-6. Stem Cell Res Ther. 2021. PMID: 33407902 Free PMC article. Review.
-
Rheumatoid Synovial Fluid and Acidic Extracellular pH Modulate the Immunomodulatory Activity of Urine-Derived Stem Cells.Int J Mol Sci. 2023 Nov 1;24(21):15856. doi: 10.3390/ijms242115856. Int J Mol Sci. 2023. PMID: 37958839 Free PMC article.
-
Human Amniotic Epithelial Cells and Their Derived Exosomes Protect Against Cisplatin-Induced Acute Kidney Injury Without Compromising Its Antitumor Activity in Mice.Front Cell Dev Biol. 2022 Feb 3;9:752053. doi: 10.3389/fcell.2021.752053. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35186944 Free PMC article.
-
Fibroblasts as Turned Agents in Cancer Progression.Cancers (Basel). 2023 Mar 28;15(7):2014. doi: 10.3390/cancers15072014. Cancers (Basel). 2023. PMID: 37046676 Free PMC article. Review.
References
-
- Martins D., Schmitt F. Microenvironment in breast tumorigenesis: Friend or foe? Histol. Histopathol. 2018;34:18021. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

