Ebola virus disease
- PMID: 32080199
- PMCID: PMC7223853
- DOI: 10.1038/s41572-020-0147-3
Ebola virus disease
Abstract
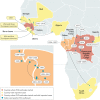
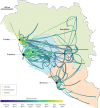
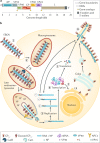
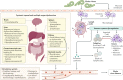
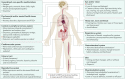
Ebola virus disease (EVD) is a severe and frequently lethal disease caused by Ebola virus (EBOV). EVD outbreaks typically start from a single case of probable zoonotic transmission, followed by human-to-human transmission via direct contact or contact with infected bodily fluids or contaminated fomites. EVD has a high case-fatality rate; it is characterized by fever, gastrointestinal signs and multiple organ dysfunction syndrome. Diagnosis requires a combination of case definition and laboratory tests, typically real-time reverse transcription PCR to detect viral RNA or rapid diagnostic tests based on immunoassays to detect EBOV antigens. Recent advances in medical countermeasure research resulted in the recent approval of an EBOV-targeted vaccine by European and US regulatory agencies. The results of a randomized clinical trial of investigational therapeutics for EVD demonstrated survival benefits from two monoclonal antibody products targeting the EBOV membrane glycoprotein. New observations emerging from the unprecedented 2013-2016 Western African EVD outbreak (the largest in history) and the ongoing EVD outbreak in the Democratic Republic of the Congo have substantially improved the understanding of EVD and viral persistence in survivors of EVD, resulting in new strategies toward prevention of infection and optimization of clinical management, acute illness outcomes and attendance to the clinical care needs of patients.
Conflict of interest statement
All authors declare no competing interests.
Figures


Similar articles
-
Ebola virus disease in the Democratic Republic of Congo.N Engl J Med. 2014 Nov 27;371(22):2083-91. doi: 10.1056/NEJMoa1411099. Epub 2014 Oct 15. N Engl J Med. 2014. PMID: 25317743
-
Delayed recognition of Ebola virus disease is associated with longer and larger outbreaks.Emerg Microbes Infect. 2020 Feb 4;9(1):291-301. doi: 10.1080/22221751.2020.1722036. eCollection 2020. Emerg Microbes Infect. 2020. PMID: 32013784 Free PMC article.
-
Atypical Ebola Virus Disease in a Nonhuman Primate following Monoclonal Antibody Treatment Is Associated with Glycoprotein Mutations within the Fusion Loop.mBio. 2021 Jan 12;12(1):e01438-20. doi: 10.1128/mBio.01438-20. mBio. 2021. PMID: 33436428 Free PMC article.
-
Treatment of ebola virus disease.Pharmacotherapy. 2015 Jan;35(1):43-53. doi: 10.1002/phar.1545. Pharmacotherapy. 2015. PMID: 25630412 Review.
-
Ebola virus disease: A narrative review.Microb Pathog. 2023 Aug;181:106213. doi: 10.1016/j.micpath.2023.106213. Epub 2023 Jun 23. Microb Pathog. 2023. PMID: 37355146 Review.
Cited by
-
Constructing, validating, and updating machine learning models to predict survival in children with Ebola Virus Disease.PLoS Negl Trop Dis. 2022 Oct 12;16(10):e0010789. doi: 10.1371/journal.pntd.0010789. eCollection 2022 Oct. PLoS Negl Trop Dis. 2022. PMID: 36223331 Free PMC article.
-
Gene expression analysis identifies hub genes and pathways distinguishing fatal from survivor outcomes of Ebola virus disease.FASEB Bioadv. 2024 Jul 29;6(9):298-310. doi: 10.1096/fba.2024-00055. eCollection 2024 Sep. FASEB Bioadv. 2024. PMID: 39399477 Free PMC article.
-
Global blood miRNA profiling unravels early signatures of immunogenicity of Ebola vaccine rVSVΔG-ZEBOV-GP.iScience. 2023 Nov 23;26(12):108574. doi: 10.1016/j.isci.2023.108574. eCollection 2023 Dec 15. iScience. 2023. PMID: 38162033 Free PMC article.
-
Navigating the Complex Landscape of Ebola Infection Treatment: A Review of Emerging Pharmacological Approaches.Infect Dis Ther. 2024 Jan;13(1):21-55. doi: 10.1007/s40121-023-00913-y. Epub 2024 Jan 19. Infect Dis Ther. 2024. PMID: 38240994 Free PMC article. Review.
-
Species-specific immunogenicity and protective efficacy of a vesicular stomatitis virus-based Sudan virus vaccine: a challenge study in macaques.Lancet Microbe. 2023 Mar;4(3):e171-e178. doi: 10.1016/S2666-5247(23)00001-0. Epub 2023 Feb 2. Lancet Microbe. 2023. PMID: 36739878 Free PMC article.
References
-
- Kuhn, J. H., Amarasinghe, G. & Perry, D. L. in Fields Virology: Emerging Viruses 7th edn Ch. 12 (eds Sean P. J. Whelan, Peter M. Howley, & David M. Knipe) in the press (Wolters Kluwer, 2020).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

