Rationale and design of the POLEM trial: avelumab plus fluoropyrimidine-based chemotherapy as adjuvant treatment for stage III mismatch repair deficient or POLE exonuclease domain mutant colon cancer: a phase III randomised study
- PMID: 32079623
- PMCID: PMC7046393
- DOI: 10.1136/esmoopen-2019-000638
Rationale and design of the POLEM trial: avelumab plus fluoropyrimidine-based chemotherapy as adjuvant treatment for stage III mismatch repair deficient or POLE exonuclease domain mutant colon cancer: a phase III randomised study
Abstract
Background: 10%-15% of early-stage colon cancers harbour either deficient mismatch repair (dMMR), microsatellite instability high (MSI-H) or POLE exonuclease domain mutations, and are characterised by high tumour mutational burden and increased lymphocytic infiltrate. Metastatic dMMR colon cancers are highly sensitive to immune checkpoint inhibition, and recent data show POLE-mutant tumours are similarly responsive. We are conducting a phase III randomised trial to determine if the addition of the anti-PD-L1 antibody avelumab following adjuvant chemotherapy improves disease-free survival (DFS) in patients with stage III dMMR/MSI-H or POLE mutant colon cancer and is a cost-effective approach for the UK National Health Service (NHS).
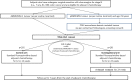
Methods: We are recruiting patients with completely resected, stage III colon cancer confirmed to have dMMR/MSI-H, locally or POLE exonuclease domain mutation on central testing. Eligible patients are randomised in a 1:1 ratio to standard fluoropyrimidine-based chemotherapy (capecitabine, oxaliplatin for 12 weeks or capecitabine for 24 weeks) or chemotherapy, followed by avelumab (10 mg/kg, 2 weekly for 24 weeks). Stratification is by chemotherapy received and MMR/MSI-H status. The primary endpoint is DFS. Secondary endpoints include overall survival, toxicity, quality of life and health resource use. The 3-year DFS rate in the control arm is expected to be ~75%. Avelumab is expected to improve the 3-year DFS rate by 12% (ie, 87%). Target accrual is 402 patients, which provides 80% power to detect an HR of 0.48 for DFS at a two-sided alpha of 0.05. This national, multicentre phase III trial is sponsored by the Royal Marsden NHS Foundation Trust and it is anticipated that approximately 40 centres in the UK will participate. This study opened to recruitment in August 2018.
Trial registration number: NCT03827044.
Keywords: POLE mutation; adjuvant therapy; colon cancer; microsatellite instability; mismatch repair.
© Author (s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ on behalf of the European Society for Medical Oncology.
Conflict of interest statement
Competing interests: IC has received advisory board fees from AstraZeneca, Bayer, Bristol Meyers Squibb, Eli-Lilly, Five Prime Therapeutics, Merck Serono, MSD, Pierre Fabre, Oncologie International and Roche; received research funding from Eli-Lilly, Janssen-Cilag, Merck Serono and Sanofi Oncology and honoraria from Eli-Lilly outside the submitted work. NS has received research funding from AstraZeneca, Bristol-Myers Squibb, Merck Serono and honoraria from AstraZeneca. DNC has research funding from Amgen, Sanofi, Merrimack, AZ, Celgene, MedImmune, Bayer, 4SC, Clovis, Eli Lilly, Janssen and Merck. TD has research funding from Merck Serono/Pfizer, honorarium/advisory board fees from Bayer, MSD, Sanofi Aventis, Amgen, Servier and Everything Genetic.
Figures
Similar articles
-
A Phase II Study of Avelumab Monotherapy in Patients with Mismatch Repair-Deficient/Microsatellite Instability-High or POLE-Mutated Metastatic or Unresectable Colorectal Cancer.Cancer Res Treat. 2020 Oct;52(4):1135-1144. doi: 10.4143/crt.2020.218. Epub 2020 Apr 24. Cancer Res Treat. 2020. PMID: 32340084 Free PMC article. Clinical Trial.
-
Prognosis of microsatellite instability and/or mismatch repair deficiency stage III colon cancer patients after disease recurrence following adjuvant treatment: results of an ACCENT pooled analysis of seven studies.Ann Oncol. 2019 Sep 1;30(9):1466-1471. doi: 10.1093/annonc/mdz208. Ann Oncol. 2019. PMID: 31268130 Free PMC article. Clinical Trial.
-
DNA mismatch repair status and colon cancer recurrence and survival in clinical trials of 5-fluorouracil-based adjuvant therapy.J Natl Cancer Inst. 2011 Jun 8;103(11):863-75. doi: 10.1093/jnci/djr153. Epub 2011 May 19. J Natl Cancer Inst. 2011. PMID: 21597022 Free PMC article.
-
Deficient mismatch repair/microsatellite unstable colorectal cancer: Diagnosis, prognosis and treatment.Eur J Cancer. 2022 Nov;175:136-157. doi: 10.1016/j.ejca.2022.07.020. Epub 2022 Sep 14. Eur J Cancer. 2022. PMID: 36115290 Review.
-
Adjuvant treatment of colon cancer with microsatellite instability - the state of the art.Crit Rev Oncol Hematol. 2022 Jan;169:103537. doi: 10.1016/j.critrevonc.2021.103537. Epub 2021 Nov 18. Crit Rev Oncol Hematol. 2022. PMID: 34801698 Review.
Cited by
-
Emerging evidence of immunotherapy for colorectal cancer.Ann Gastroenterol Surg. 2022 Nov 8;7(2):216-224. doi: 10.1002/ags3.12633. eCollection 2023 Mar. Ann Gastroenterol Surg. 2022. PMID: 36998297 Free PMC article. Review.
-
LAG-3 Expression Predicts Outcome in Stage II Colon Cancer.J Pers Med. 2021 Jul 30;11(8):749. doi: 10.3390/jpm11080749. J Pers Med. 2021. PMID: 34442393 Free PMC article.
-
Prognostic and Predictive Values of Mismatch Repair Deficiency in Non-Metastatic Colorectal Cancer.Cancers (Basel). 2021 Jan 15;13(2):300. doi: 10.3390/cancers13020300. Cancers (Basel). 2021. PMID: 33467526 Free PMC article. Review.
-
Is the combination of immunotherapy with conventional chemotherapy the key to increase the efficacy of colorectal cancer treatment?World J Gastrointest Oncol. 2023 Feb 15;15(2):251-267. doi: 10.4251/wjgo.v15.i2.251. World J Gastrointest Oncol. 2023. PMID: 36908325 Free PMC article. Review.
-
Immune-Checkpoint Inhibitors (ICIs) in Metastatic Colorectal Cancer (mCRC) Patients beyond Microsatellite Instability.Cancers (Basel). 2022 Oct 11;14(20):4974. doi: 10.3390/cancers14204974. Cancers (Basel). 2022. PMID: 36291761 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials