Novel Sustained-Release Drug Delivery System for Dry Eye Therapy by Rebamipide Nanoparticles
- PMID: 32075200
- PMCID: PMC7076486
- DOI: 10.3390/pharmaceutics12020155
Novel Sustained-Release Drug Delivery System for Dry Eye Therapy by Rebamipide Nanoparticles
Abstract
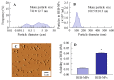
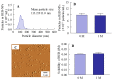
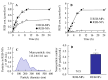
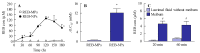
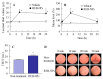
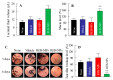
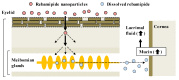
The commercially available rebamipide ophthalmic suspension (CA-REB) was approved for clinical use in patients with dry eye; however, the residence time on the ocular surface for the traditional formulations is short, since the drug is removed from the ocular surface through the nasolacrimal duct. In this study, we designed a novel sustained-release drug delivery system (DDS) for dry eye therapy by rebamipide nanoparticles. The rebamipide solid nanoparticle-based ophthalmic formulation (REB-NPs) was prepared by a bead mill using additives (2-hydroxypropyl-β-cyclodextrin and methylcellulose) and a gel base (carbopol). The rebamipide particles formed are ellipsoid, with a particle size in the range of 40-200 nm. The rebamipide in the REB-NPs applied to eyelids was delivered into the lacrimal fluid through the meibomian glands, and sustained drug release was observed in comparison with CA-REB. Moreover, the REB-NPs increased the mucin levels in the lacrimal fluid and healed tear film breakup levels in an N-acetylcysteine-treated rabbit model. The information about this novel DDS route and creation of a nano-formulation can be used to design further studies aimed at therapy for dry eye.
Keywords: dry eye; eyelid; mucin; rebamipide; sustained delivery system.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Hydrogel Formulations Incorporating Drug Nanocrystals Enhance the Therapeutic Effect of Rebamipide in a Hamster Model for Oral Mucositis.Pharmaceutics. 2020 Jun 9;12(6):532. doi: 10.3390/pharmaceutics12060532. Pharmaceutics. 2020. PMID: 32527029 Free PMC article.
-
Development of Sustained-Release Ophthalmic Formulation Based on Tranilast Solid Nanoparticles.Materials (Basel). 2020 Apr 3;13(7):1675. doi: 10.3390/ma13071675. Materials (Basel). 2020. PMID: 32260210 Free PMC article.
-
Copolymerized Polymers Based on Cyclodextrins and Cationic Groups Enhance Therapeutic Effect of Rebamipide in the N-Acetylcysteine-Treated Dry Eye Model.Drug Des Devel Ther. 2024 Sep 27;18:4345-4358. doi: 10.2147/DDDT.S469445. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39359484 Free PMC article.
-
The Efficacy and Safety of Rebamipide Ophthalmic Suspension (OPC-12759) in Patients with Dry Eye Disease: A Systematic Review of Randomized Controlled Trials.J Clin Med. 2023 Nov 17;12(22):7155. doi: 10.3390/jcm12227155. J Clin Med. 2023. PMID: 38002767 Free PMC article. Review.
-
Rebamipide ophthalmic suspension for the treatment of dry eye syndrome: a critical appraisal.Clin Ophthalmol. 2014 May 30;8:1003-10. doi: 10.2147/OPTH.S40798. eCollection 2014. Clin Ophthalmol. 2014. PMID: 24940041 Free PMC article. Review.
Cited by
-
Ophthalmic In Situ Gelling System Containing Lanosterol Nanoparticles Delays Collapse of Lens Structure in Shumiya Cataract Rats.Pharmaceutics. 2020 Jul 4;12(7):629. doi: 10.3390/pharmaceutics12070629. Pharmaceutics. 2020. PMID: 32635523 Free PMC article.
-
Engineering Advanced Drug Delivery Systems for Dry Eye: A Review.Bioengineering (Basel). 2022 Dec 31;10(1):53. doi: 10.3390/bioengineering10010053. Bioengineering (Basel). 2022. PMID: 36671625 Free PMC article. Review.
-
MPC Polymer Promotes Recovery from Dry Eye via Stabilization of the Ocular Surface.Pharmaceutics. 2021 Jan 27;13(2):168. doi: 10.3390/pharmaceutics13020168. Pharmaceutics. 2021. PMID: 33513827 Free PMC article.
-
Hydrogel Formulations Incorporating Drug Nanocrystals Enhance the Therapeutic Effect of Rebamipide in a Hamster Model for Oral Mucositis.Pharmaceutics. 2020 Jun 9;12(6):532. doi: 10.3390/pharmaceutics12060532. Pharmaceutics. 2020. PMID: 32527029 Free PMC article.
-
Development of Sustained-Release Ophthalmic Formulation Based on Tranilast Solid Nanoparticles.Materials (Basel). 2020 Apr 3;13(7):1675. doi: 10.3390/ma13071675. Materials (Basel). 2020. PMID: 32260210 Free PMC article.
References
-
- Lemp M.A., Foulks G.N. The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop. Ocul. Surf. 2007;5:75–92. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

