Validation of a hierarchical algorithm to define chronic liver disease and cirrhosis etiology in administrative healthcare data
- PMID: 32069337
- PMCID: PMC7028265
- DOI: 10.1371/journal.pone.0229218
Validation of a hierarchical algorithm to define chronic liver disease and cirrhosis etiology in administrative healthcare data
Abstract
Background and aims: Chronic liver disease (CLD) and cirrhosis are leading causes of death globally with the burden of disease rising significantly over the past several decades. Defining the etiology of liver disease is important for understanding liver disease epidemiology, healthcare planning, and outcomes. The aim of this study was to validate a hierarchical algorithm for CLD and cirrhosis etiology in administrative healthcare data.
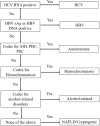
Methods: Consecutive patients with CLD or cirrhosis attending an outpatient hepatology clinic in Ontario, Canada from 05/01/2013-08/31/2013 underwent detailed chart abstraction. Gold standard liver disease etiology was determined by an attending hepatologist as hepatitis C (HCV), hepatitis B (HBV), alcohol-related, non-alcoholic fatty liver disease (NAFLD)/cryptogenic, autoimmune or hemochromatosis. Individual data was linked to routinely collected administrative healthcare data at ICES. Diagnostic accuracy of a hierarchical algorithm incorporating both laboratory and administrative codes to define etiology was evaluated by calculating sensitivity, specificity, positive (PPV) and negative predictive values (NPV), and kappa's agreement.
Results: 442 individuals underwent chart abstraction (median age 53 years, 53% cirrhosis, 45% HCV, 26% NAFLD, 10% alcohol-related). In patients with cirrhosis, the algorithm had adequate sensitivity/PPV (>75%) and excellent specificity/NPV (>90%) for all etiologies. In those without cirrhosis, the algorithm was excellent for all etiologies except for hemochromatosis and autoimmune diseases.
Conclusions: A hierarchical algorithm incorporating laboratory and administrative coding can accurately define cirrhosis etiology in routinely collected healthcare data. These results should facilitate health services research in this growing patient population.
Conflict of interest statement
I have read the journal’s policy and the authors of the manuscript have the following competing interests: Jennifer A. Flemming declares investigator-driven research support received from Gilead Sciences Canada, as well as speaker fees from Gilead Sciences, AbbVie, and Lupin Pharmaceuticals. The commercial affiliations of author JAF had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript and only provided financial support in the form of author’s salaries. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
Similar articles
-
Identifying cirrhosis, decompensated cirrhosis and hepatocellular carcinoma in health administrative data: A validation study.PLoS One. 2018 Aug 22;13(8):e0201120. doi: 10.1371/journal.pone.0201120. eCollection 2018. PLoS One. 2018. PMID: 30133446 Free PMC article.
-
Validation of a coding algorithm to identify patients with hepatocellular carcinoma in an administrative database.Pharmacoepidemiol Drug Saf. 2013 Jan;22(1):103-7. doi: 10.1002/pds.3367. Epub 2012 Nov 4. Pharmacoepidemiol Drug Saf. 2013. PMID: 23124932 Free PMC article.
-
Study of liver cirrhosis over ten consecutive years in Southern China.World J Gastroenterol. 2014 Oct 7;20(37):13546-55. doi: 10.3748/wjg.v20.i37.13546. World J Gastroenterol. 2014. PMID: 25309085 Free PMC article.
-
Hepatocellular carcinoma in the post-hepatitis C virus era: Should we change the paradigm?World J Gastroenterol. 2019 Aug 7;25(29):3929-3940. doi: 10.3748/wjg.v25.i29.3929. World J Gastroenterol. 2019. PMID: 31413528 Free PMC article. Review.
-
Hepatocellular carcinoma in non-alcoholic steatohepatitis without cirrhosis.World J Gastroenterol. 2023 Jan 14;29(2):343-356. doi: 10.3748/wjg.v29.i2.343. World J Gastroenterol. 2023. PMID: 36687125 Free PMC article. Review.
Cited by
-
Impact of the COVID-19 Pandemic on Hospitalizations for Alcoholic Hepatitis or Cirrhosis in Alberta, Canada.Clin Gastroenterol Hepatol. 2022 May;20(5):e1170-e1179. doi: 10.1016/j.cgh.2021.10.030. Epub 2021 Oct 26. Clin Gastroenterol Hepatol. 2022. PMID: 34715379 Free PMC article.
-
Colorectal Cancer in Individuals with Cirrhosis: A Population-Based Study Assessing Practice Patterns, Outcomes, and Predictors of Survival.Curr Oncol. 2023 Oct 30;30(11):9530-9541. doi: 10.3390/curroncol30110690. Curr Oncol. 2023. PMID: 37999110 Free PMC article.
-
Associations between modest reductions in kidney function and adverse outcomes in young adults: retrospective, population based cohort study.BMJ. 2023 Jun 22;381:e075062. doi: 10.1136/bmj-2023-075062. BMJ. 2023. PMID: 37353230 Free PMC article.
-
External Validation of the VOCAL-Penn Cirrhosis Surgical Risk Score in 2 Large, Independent Health Systems.Liver Transpl. 2021 Jul;27(7):961-970. doi: 10.1002/lt.26060. Liver Transpl. 2021. PMID: 33788365 Free PMC article.
-
Characterizing the cascade of care for hepatitis C virus infection among Status First Nations peoples in Ontario: a retrospective cohort study.CMAJ. 2023 Apr 11;195(14):E499-E512. doi: 10.1503/cmaj.220717. CMAJ. 2023. PMID: 37040993 Free PMC article.
References
-
- Heron M. National Vital Statistics Reports Volume 65, Number 5 June 30, 2016 Deaths: Leading Causes for 2014. 2016;65(5). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous