Long-Term Outcomes and Responses to Retreatment in Patients With Melanoma Treated With PD-1 Blockade
- PMID: 32053428
- PMCID: PMC7238490
- DOI: 10.1200/JCO.19.01464
Long-Term Outcomes and Responses to Retreatment in Patients With Melanoma Treated With PD-1 Blockade
Abstract
Purpose: To analyze long-term outcomes after treatment discontinuation of anti-programmed death-1 (anti-PD-1) therapy in a cohort of patients with melanoma with the longest follow-up yet available to our knowledge, including a majority of patients treated outside of a clinical trial. We also assessed efficacy of retreatment with anti-PD-1 therapy with or without ipilimumab in relapsing patients.
Methods: We retrospectively analyzed all patients with nonuveal, unresectable stage III/IV melanoma treated with single-agent anti-PD-1 therapy at Memorial Sloan Kettering from 2009-2018 who had discontinued treatment and had at least 3 months of follow-up after discontinuation (n = 396). Overall survival for patients with complete response (CR) was calculated from time of CR. Time to treatment failure for patients with CR was time from CR to the next melanoma treatment or death.
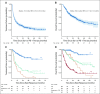
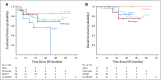
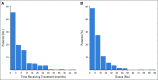
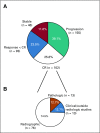
Results: CRs were seen in 102 of 396 patients (25.8%). The median number of months of treatment after CR was zero (range, stopped before CR to 26 months after CR). With a median follow-up of 21.1 months from time of CR in patients who did not relapse, the probability of being alive and not needing additional melanoma therapy at 3 years was 72.1%. There was no significant association between treatment duration and relapse risk. In multivariable analysis, CR was associated with M1b disease and cutaneous versus mucosal or acral primaries. Among the 78 patients (of 396) retreated after disease progression, response was seen in 5 of 34 retreated patients with single-agent anti-PD-1 therapy and 11 of 44 patients escalated to anti-PD-1 plus ipilimumab.
Conclusion: In our cohort, most patients discontinued treatment at the time of CR. Most CRs were durable but the probability of treatment failure was 27% at 3 years. Responses to retreatment were infrequent. The optimal duration of treatment after CR is not yet established.
Figures

Comment in
-
Is It Safe to Stop Anti-PD-1 Immunotherapy in Patients With Metastatic Melanoma Who Achieve a Complete Response?J Clin Oncol. 2020 May 20;38(15):1645-1647. doi: 10.1200/JCO.20.00136. Epub 2020 Feb 25. J Clin Oncol. 2020. PMID: 32097093 Free PMC article. No abstract available.
Similar articles
-
Discontinuation of anti-PD-1 antibody therapy in the absence of disease progression or treatment limiting toxicity: clinical outcomes in advanced melanoma.Ann Oncol. 2019 Jul 1;30(7):1154-1161. doi: 10.1093/annonc/mdz110. Ann Oncol. 2019. PMID: 30923820
-
Real-world experience with elective discontinuation of PD-1 inhibitors at 1 year in patients with metastatic melanoma.J Immunother Cancer. 2021 Jan;9(1):e001781. doi: 10.1136/jitc-2020-001781. J Immunother Cancer. 2021. PMID: 33500258 Free PMC article.
-
Discontinuation of anti-PD1 in advanced melanoma: an observational retrospective study from the Italian Melanoma Intergroup.Eur J Cancer. 2023 Jul;187:25-35. doi: 10.1016/j.ejca.2023.03.020. Epub 2023 Mar 23. Eur J Cancer. 2023. PMID: 37099946
-
The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma.Clin Ther. 2015 Apr 1;37(4):764-82. doi: 10.1016/j.clinthera.2015.02.018. Epub 2015 Mar 29. Clin Ther. 2015. PMID: 25823918 Free PMC article. Review.
-
Immunotherapy for advanced melanoma: current situation in Japan.Jpn J Clin Oncol. 2021 Jan 1;51(1):3-9. doi: 10.1093/jjco/hyaa188. Jpn J Clin Oncol. 2021. PMID: 33140101 Review.
Cited by
-
Efficacy and safety of lifileucel, a one-time autologous tumor-infiltrating lymphocyte (TIL) cell therapy, in patients with advanced melanoma after progression on immune checkpoint inhibitors and targeted therapies: pooled analysis of consecutive cohorts of the C-144-01 study.J Immunother Cancer. 2022 Dec;10(12):e005755. doi: 10.1136/jitc-2022-005755. J Immunother Cancer. 2022. PMID: 36600653 Free PMC article. Clinical Trial.
-
Management of early melanoma recurrence despite adjuvant anti-PD-1 antibody therapy☆.Ann Oncol. 2020 Aug;31(8):1075-1082. doi: 10.1016/j.annonc.2020.04.471. Epub 2020 May 6. Ann Oncol. 2020. PMID: 32387454 Free PMC article.
-
Three-year survival, correlates and salvage therapies in patients receiving first-line pembrolizumab for advanced Merkel cell carcinoma.J Immunother Cancer. 2021 Apr;9(4):e002478. doi: 10.1136/jitc-2021-002478. J Immunother Cancer. 2021. PMID: 33879601 Free PMC article. Clinical Trial.
-
Development and pharmacokinetic assessment of a fully canine anti-PD-1 monoclonal antibody for comparative translational research in dogs with spontaneous tumors.MAbs. 2023 Jan-Dec;15(1):2287250. doi: 10.1080/19420862.2023.2287250. Epub 2023 Dec 4. MAbs. 2023. PMID: 38047502 Free PMC article.
-
Immune checkpoint inhibitor (ICI)-based treatment beyond progression with prior immunotherapy in patients with stage IV non-small cell lung cancer: a retrospective study.Transl Lung Cancer Res. 2022 Jun;11(6):1027-1037. doi: 10.21037/tlcr-22-376. Transl Lung Cancer Res. 2022. PMID: 35832458 Free PMC article.
References
-
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. - PubMed
-
- Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. - PubMed
-
- Robert C, Ribas A, Hamid O, et al. Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanoma. J Clin Oncol. 2018;36:1668–1674. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

