Morphine-induced respiratory depression is independent of β-arrestin2 signalling
- PMID: 32052419
- PMCID: PMC7280004
- DOI: 10.1111/bph.15004
Morphine-induced respiratory depression is independent of β-arrestin2 signalling
Abstract
Background and purpose: GPCRs can signal through both G proteins and β-arrestin2. For the μ-opioid receptor, early experimental evidence from a single study suggested that G protein signalling mediates analgesia, whereas β-arrestin2 signalling mediates respiratory depression and constipation. Consequently, for more than a decade, much research effort has been focused on developing biased μ-opioid agonists that preferentially target G protein signalling over β-arrestin signalling, as it was believed that such drugs would be analgesics devoid of respiratory depressant activity. However, the prototypical compounds that have been developed based on this concept have so far failed in clinical and preclinical development.
Experimental approach: The present study was set up to re-examine opioid-induced respiratory depression in β-arrestin2 knockout mice. To this end, a consortium was formed consisting of three different laboratories located in different countries to evaluate independently opioid-induced respiratory depression.
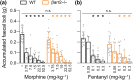
Key results: Our consensus results unequivocally demonstrate that the prototypical μ-opioid agonist morphine (3.75-100 mg·kg-1 s.c. or 3-30 mg·kg-1 i.p.) as well as the potent opioid fentanyl (0.05-0.35 mg·kg-1 s.c.) do indeed induce respiratory depression and constipation in β-arrestin2 knockout mice in a dose-dependent manner indistinguishable from that observed in wild-type mice.
Conclusion and implications: Our findings do not support the original suggestion that β-arrestin2 signalling plays a key role in opioid-induced respiratory depression and call into question the concept of developing G protein-biased μ-opioid receptor agonists as a strategy for the development of safer opioid analgesic drugs.
© 2020 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Comment in
-
Commentary on "Morphine-induced respiratory depression is independent of β-arrestin2 signalling".Br J Pharmacol. 2020 Jul;177(13):2904-2905. doi: 10.1111/bph.15090. Epub 2020 May 27. Br J Pharmacol. 2020. PMID: 32462692 Free PMC article. No abstract available.
Similar articles
-
The μ-opioid receptor-mediated Gi/o protein and β-arrestin2 signaling pathways both contribute to morphine-induced side effects.Eur J Pharmacol. 2024 Mar 5;966:176333. doi: 10.1016/j.ejphar.2024.176333. Epub 2024 Jan 24. Eur J Pharmacol. 2024. PMID: 38278466
-
Intracerebroventricular administration of CYX-6, a potent μ-opioid receptor agonist, a δ- and κ-opioid receptor antagonist and a biased ligand at μ, δ & κ-opioid receptors, evokes antinociception with minimal constipation and respiratory depression in rats in contrast to morphine.Eur J Pharmacol. 2020 Mar 15;871:172918. doi: 10.1016/j.ejphar.2020.172918. Epub 2020 Jan 17. Eur J Pharmacol. 2020. PMID: 31958457
-
In vitro profiling of opioid ligands using the cAMP formation inhibition assay and the β-arrestin2 recruitment assay: No two ligands have the same profile.Eur J Pharmacol. 2020 Apr 5;872:172947. doi: 10.1016/j.ejphar.2020.172947. Epub 2020 Jan 25. Eur J Pharmacol. 2020. PMID: 31991138
-
Novel Opioid Receptor Agonists with Reduced Morphine-like Side Effects.Mini Rev Med Chem. 2018;18(19):1603-1610. doi: 10.2174/1389557518666180716124336. Mini Rev Med Chem. 2018. PMID: 30009707 Review.
-
Critical Assessment of G Protein-Biased Agonism at the μ-Opioid Receptor.Trends Pharmacol Sci. 2020 Dec;41(12):947-959. doi: 10.1016/j.tips.2020.09.009. Epub 2020 Oct 20. Trends Pharmacol Sci. 2020. PMID: 33097283 Review.
Cited by
-
Direct interrogation of context-dependent GPCR activity with a universal biosensor platform.bioRxiv [Preprint]. 2024 Jan 2:2024.01.02.573921. doi: 10.1101/2024.01.02.573921. bioRxiv. 2024. Update in: Cell. 2024 Mar 14;187(6):1527-1546.e25. doi: 10.1016/j.cell.2024.01.028 PMID: 38260348 Free PMC article. Updated. Preprint.
-
Allosteric modulation of G protein-coupled receptors as a novel therapeutic strategy in neuropathic pain.Acta Pharm Sin B. 2024 Jan;14(1):67-86. doi: 10.1016/j.apsb.2023.07.020. Epub 2023 Jul 21. Acta Pharm Sin B. 2024. PMID: 38239234 Free PMC article. Review.
-
Heroin and its metabolites: relevance to heroin use disorder.Transl Psychiatry. 2023 Apr 8;13(1):120. doi: 10.1038/s41398-023-02406-5. Transl Psychiatry. 2023. PMID: 37031205 Free PMC article. Review.
-
Oxycodone in the Opioid Epidemic: High 'Liking', 'Wanting', and Abuse Liability.Cell Mol Neurobiol. 2021 Jul;41(5):899-926. doi: 10.1007/s10571-020-01013-y. Epub 2020 Nov 27. Cell Mol Neurobiol. 2021. PMID: 33245509 Free PMC article. Review.
-
Positive allosteric modulation of the mu-opioid receptor produces analgesia with reduced side effects.Proc Natl Acad Sci U S A. 2021 Apr 20;118(16):e2000017118. doi: 10.1073/pnas.2000017118. Proc Natl Acad Sci U S A. 2021. PMID: 33846240 Free PMC article.
References
-
- Alexander, S. P. H. , Roberts, R. E. , Broughton, B. R. S. , Sobey, C. G. , George, C. H. , & Stanford, S. C. (2018). Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology. British Journal of Pharmacology, 175, 407–411. - PMC - PubMed
-
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

