The Role of TRPC1 in Modulating Cancer Progression
- PMID: 32046188
- PMCID: PMC7072717
- DOI: 10.3390/cells9020388
The Role of TRPC1 in Modulating Cancer Progression
Abstract
Calcium ions (Ca2+) play an important role as second messengers in regulating a plethora of physiological and pathological processes, including the progression of cancer. Several selective and non-selective Ca2+-permeable ion channels are implicated in mediating Ca2+ signaling in cancer cells. In this review, we are focusing on TRPC1, a member of the TRP protein superfamily and a potential modulator of store-operated Ca2+ entry (SOCE) pathways. While TRPC1 is ubiquitously expressed in most tissues, its dysregulated activity may contribute to the hallmarks of various types of cancers, including breast cancer, pancreatic cancer, glioblastoma multiforme, lung cancer, hepatic cancer, multiple myeloma, and thyroid cancer. A range of pharmacological and genetic tools have been developed to address the functional role of TRPC1 in cancer. Interestingly, the unique role of TRPC1 has elevated this channel as a promising target for modulation both in terms of pharmacological inhibition leading to suppression of tumor growth and metastasis, as well as for agonistic strategies eliciting Ca2+overload and cell death in aggressive metastatic tumor cells.
Keywords: EMT; SOCE; TRPC1; cancer progression.
Conflict of interest statement
L.H. is a co-founder of Modulation Therapeutics Inc. which has a license to MTI-101 for treatment of cancer. The other authors declare no conflict of interest.
Figures
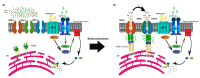
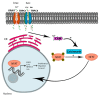
Similar articles
-
TRPC1 and ORAI1 channels in colon cancer.Cell Calcium. 2019 Jul;81:59-66. doi: 10.1016/j.ceca.2019.06.003. Epub 2019 Jun 7. Cell Calcium. 2019. PMID: 31203007 Review.
-
STIM-TRP Pathways and Microdomain Organization: Contribution of TRPC1 in Store-Operated Ca2+ Entry: Impact on Ca2+ Signaling and Cell Function.Adv Exp Med Biol. 2017;993:159-188. doi: 10.1007/978-3-319-57732-6_9. Adv Exp Med Biol. 2017. PMID: 28900914 Review.
-
TRPC Channels in the SOCE Scenario.Cells. 2020 Jan 5;9(1):126. doi: 10.3390/cells9010126. Cells. 2020. PMID: 31948094 Free PMC article. Review.
-
TRPC1 functions as a store-operated Ca2+ channel in intestinal epithelial cells and regulates early mucosal restitution after wounding.Am J Physiol Gastrointest Liver Physiol. 2006 Apr;290(4):G782-92. doi: 10.1152/ajpgi.00441.2005. Epub 2005 Nov 10. Am J Physiol Gastrointest Liver Physiol. 2006. PMID: 16282360
-
A role for calcium in the regulation of ATP-binding cassette, sub-family C, member 3 (ABCC3) gene expression in a model of epidermal growth factor-mediated breast cancer epithelial-mesenchymal transition.Biochem Biophys Res Commun. 2015 Mar 13;458(3):509-514. doi: 10.1016/j.bbrc.2015.01.141. Epub 2015 Feb 7. Biochem Biophys Res Commun. 2015. PMID: 25666946
Cited by
-
Transient Receptor Potential Canonical Channels in Health and Disease: A 2020 Update.Cells. 2021 Feb 25;10(3):496. doi: 10.3390/cells10030496. Cells. 2021. PMID: 33668918 Free PMC article.
-
Mitochondrial Ca2+ Signaling in Health, Disease and Therapy.Cells. 2021 May 25;10(6):1317. doi: 10.3390/cells10061317. Cells. 2021. PMID: 34070562 Free PMC article. Review.
-
Ion Channel Involvement in Tumor Drug Resistance.J Pers Med. 2022 Feb 3;12(2):210. doi: 10.3390/jpm12020210. J Pers Med. 2022. PMID: 35207698 Free PMC article. Review.
-
Targeting transient receptor potential canonical 1 reduces non‑small cell lung cancer chemoresistance and stemness via inhibition of PI3K/AKT signaling.Oncol Lett. 2023 Apr 13;25(6):224. doi: 10.3892/ol.2023.13810. eCollection 2023 Jun. Oncol Lett. 2023. PMID: 37153044 Free PMC article.
-
TRP Channels Interactome as a Novel Therapeutic Target in Breast Cancer.Front Oncol. 2021 Jun 10;11:621614. doi: 10.3389/fonc.2021.621614. eCollection 2021. Front Oncol. 2021. PMID: 34178620 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

