Transdermal cold atmospheric plasma-mediated immune checkpoint blockade therapy
- PMID: 32029590
- PMCID: PMC7035610
- DOI: 10.1073/pnas.1917891117
Transdermal cold atmospheric plasma-mediated immune checkpoint blockade therapy
Abstract
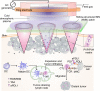
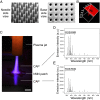
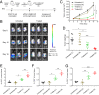
Despite the promise of immune checkpoint blockade (ICB) therapy against cancer, challenges associated with low objective response rates and severe systemic side effects still remain and limit its clinical applications. Here, we described a cold atmospheric plasma (CAP)-mediated ICB therapy integrated with microneedles (MN) for the transdermal delivery of ICB. We found that a hollow-structured MN (hMN) patch facilitates the transportation of CAP through the skin, causing tumor cell death. The release of tumor-associated antigens then promotes the maturation of dendritic cells in the tumor-draining lymph nodes, subsequently initiating T cell-mediated immune response. Anti-programmed death-ligand 1 antibody (aPDL1), an immune checkpoint inhibitor, released from the MN patch further augments the antitumor immunity. Our findings indicate that the proposed transdermal combined CAP and ICB therapy can inhibit the tumor growth of both primary tumors and distant tumors, prolonging the survival of tumor-bearing mice.
Keywords: cancer immunotherapy; cold atmospheric plasma; drug delivery; immune checkpoint blockade; microneedle.
Conflict of interest statement
Competing interest statement: G.C., Z.C., R.E.W., and Z.G. have applied for patents related to this study.
Figures

Similar articles
-
Local and Targeted Delivery of Immune Checkpoint Blockade Therapeutics.Acc Chem Res. 2020 Nov 17;53(11):2521-2533. doi: 10.1021/acs.accounts.0c00339. Epub 2020 Oct 19. Acc Chem Res. 2020. PMID: 33073988 Free PMC article.
-
Injectable cold atmospheric plasma-activated immunotherapeutic hydrogel for enhanced cancer treatment.Biomaterials. 2023 Sep;300:122189. doi: 10.1016/j.biomaterials.2023.122189. Epub 2023 Jun 1. Biomaterials. 2023. PMID: 37307777
-
Genetically Engineered Cytomembrane Nanovaccines for Cancer Immunotherapy.Adv Healthc Mater. 2024 May;13(13):e2400068. doi: 10.1002/adhm.202400068. Epub 2024 Feb 15. Adv Healthc Mater. 2024. PMID: 38320299
-
The application of nanotechnology in immune checkpoint blockade for cancer treatment.J Control Release. 2018 Nov 28;290:28-45. doi: 10.1016/j.jconrel.2018.09.026. Epub 2018 Oct 1. J Control Release. 2018. PMID: 30287266 Review.
-
FcγR-Binding Is an Important Functional Attribute for Immune Checkpoint Antibodies in Cancer Immunotherapy.Front Immunol. 2019 Feb 26;10:292. doi: 10.3389/fimmu.2019.00292. eCollection 2019. Front Immunol. 2019. PMID: 30863404 Free PMC article. Review.
Cited by
-
Organoids: new frontiers in tumor immune microenvironment research.Front Immunol. 2024 Jul 29;15:1422031. doi: 10.3389/fimmu.2024.1422031. eCollection 2024. Front Immunol. 2024. PMID: 39136020 Free PMC article. Review.
-
Cell and tissue engineering in lymph nodes for cancer immunotherapy.Adv Drug Deliv Rev. 2020;161-162:42-62. doi: 10.1016/j.addr.2020.07.023. Epub 2020 Aug 1. Adv Drug Deliv Rev. 2020. PMID: 32750376 Free PMC article.
-
Co-delivery of dendritic cell vaccine and anti-PD-1 antibody with cryomicroneedles for combinational immunotherapy.Bioeng Transl Med. 2022 Nov 27;8(5):e10457. doi: 10.1002/btm2.10457. eCollection 2023 Sep. Bioeng Transl Med. 2022. PMID: 37693072 Free PMC article.
-
Recent advances in microneedles-mediated transdermal delivery of protein and peptide drugs.Acta Pharm Sin B. 2021 Aug;11(8):2326-2343. doi: 10.1016/j.apsb.2021.03.003. Epub 2021 Mar 10. Acta Pharm Sin B. 2021. PMID: 34522590 Free PMC article. Review.
-
Versatile Ice Microneedles for Transdermal Delivery of Diverse Actives.Adv Sci (Weinh). 2021 Sep;8(17):e2101210. doi: 10.1002/advs.202101210. Epub 2021 Jul 3. Adv Sci (Weinh). 2021. PMID: 34218532 Free PMC article.
References
-
- Sharma P., Allison J. P., The future of immune checkpoint therapy. Science 348, 56–61 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

