Improved therapeutics of modified mesenchymal stem cells: an update
- PMID: 32000804
- PMCID: PMC6993499
- DOI: 10.1186/s12967-020-02234-x
Improved therapeutics of modified mesenchymal stem cells: an update
Abstract
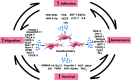
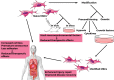
Background: Mesenchymal stromal cells (MSCs) have attracted intense interest due to their powerful intrinsic properties of self-regeneration, immunomodulation and multi-potency, as well as being readily available and easy to isolate and culture. Notwithstanding, MSC based therapy suffers reduced efficacy due to several challenges which include unfavorable microenvironmental factors in vitro and in vivo. BODY: In the quest to circumvent these challenges, several modification techniques have been applied to the naïve MSC to improve its inherent therapeutic properties. These modification approaches can be broadly divided into two groups to include genetic modification and preconditioning modification (using drugs, growth factors and other molecules). This field has witnessed great progress and continues to gather interest and novelty. We review these innovative approaches in not only maintaining, but also enhancing the inherent biological activities and therapeutics of MSCs with respect to migration, homing to target site, adhesion, survival and reduced premature senescence. We discuss the application of the improved modified MSC in some selected human diseases. Possible ways of yet better enhancing the therapeutic outcome and overcoming challenges of MSC modification in the future are also elaborated.
Conclusion: The importance of prosurvival and promigratory abilities of MSCs in their therapeutic applications can never be overemphasized. These abilities are maintained and even further enhanced via MSC modifications against the inhospitable microenvironment during culture and transplantation. This is a turning point in MSC-based therapy with promising preclinical studies and higher future prospect.
Keywords: Genetic; Mesenchymal stem cells; Modification; Preconditioning; Therapy.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Priming strategies for controlling stem cell fate: Applications and challenges in dental tissue regeneration.World J Stem Cells. 2021 Nov 26;13(11):1625-1646. doi: 10.4252/wjsc.v13.i11.1625. World J Stem Cells. 2021. PMID: 34909115 Free PMC article. Review.
-
Therapeutic gene delivery by mesenchymal stem cell for brain ischemia damage: Focus on molecular mechanisms in ischemic stroke.Cell Biochem Funct. 2024 Mar;42(2):e3957. doi: 10.1002/cbf.3957. Cell Biochem Funct. 2024. PMID: 38468129 Review.
-
Preconditioning influences mesenchymal stem cell properties in vitro and in vivo.J Cell Mol Med. 2018 Mar;22(3):1428-1442. doi: 10.1111/jcmm.13492. Epub 2018 Feb 1. J Cell Mol Med. 2018. PMID: 29392844 Free PMC article. Review.
-
Genetic modification and preconditioning strategies to enhance functionality of mesenchymal stromal cells: a clinical perspective.Expert Opin Biol Ther. 2023 Jan-Jun;23(6):461-478. doi: 10.1080/14712598.2023.2205017. Epub 2023 Apr 23. Expert Opin Biol Ther. 2023. PMID: 37073114 Review.
-
Cell adhesion and long-term survival of transplanted mesenchymal stem cells: a prerequisite for cell therapy.Oxid Med Cell Longev. 2015;2015:632902. doi: 10.1155/2015/632902. Epub 2015 Feb 2. Oxid Med Cell Longev. 2015. PMID: 25722795 Free PMC article. Review.
Cited by
-
Current Strategies and Therapeutic Applications of Mesenchymal Stem Cell-Based Drug Delivery.Pharmaceuticals (Basel). 2024 May 30;17(6):707. doi: 10.3390/ph17060707. Pharmaceuticals (Basel). 2024. PMID: 38931374 Free PMC article. Review.
-
Preconditioning mesenchymal stromal cells with flagellin enhances the anti‑inflammatory ability of their secretome against lipopolysaccharide‑induced acute lung injury.Mol Med Rep. 2020 Oct;22(4):2753-2766. doi: 10.3892/mmr.2020.11380. Epub 2020 Jul 28. Mol Med Rep. 2020. PMID: 32945411 Free PMC article.
-
Aging and Mesenchymal Stem Cells: Basic Concepts, Challenges and Strategies.Biology (Basel). 2022 Nov 18;11(11):1678. doi: 10.3390/biology11111678. Biology (Basel). 2022. PMID: 36421393 Free PMC article. Review.
-
Tacrolimus Improves Therapeutic Efficacy of Umbilical Cord Blood-Derived Mesenchymal Stem Cells in Diabetic Retinopathy by Suppressing DRP1-Mediated Mitochondrial Fission.Antioxidants (Basel). 2023 Sep 6;12(9):1727. doi: 10.3390/antiox12091727. Antioxidants (Basel). 2023. PMID: 37760030 Free PMC article.
-
Mesenchymal Stem Cell-Derived Extracellular Vesicles: An Emerging Diagnostic and Therapeutic Biomolecules for Neurodegenerative Disabilities.Biomolecules. 2023 Aug 16;13(8):1250. doi: 10.3390/biom13081250. Biomolecules. 2023. PMID: 37627315 Free PMC article. Review.
References
-
- Gee AP, Richman S, Durett A, McKenna D, Traverse J, Henry T, et al. Multicenter cell processing for cardiovascular regenerative medicine applications: the cardiovascular cell therapy research network (CCTRN) experience. Cytotherapy. 2010;12:684–691. doi: 10.3109/14653249.2010.487900. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

