Exosomes from patients with major depression cause depressive-like behaviors in mice with involvement of miR-139-5p-regulated neurogenesis
- PMID: 31986519
- PMCID: PMC7162931
- DOI: 10.1038/s41386-020-0622-2
Exosomes from patients with major depression cause depressive-like behaviors in mice with involvement of miR-139-5p-regulated neurogenesis
Abstract
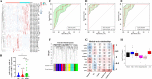
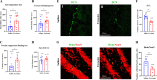
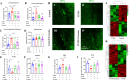
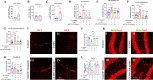
Exosomal microRNAs (miRNAs) have been suggested to participate in the pathogenesis of neuropsychiatric diseases, but their role in major depressive disorder (MDD) is unknown. We performed a genome-wide miRNA expression profiling of blood-derived exosomes from MDD patients and control subjects and revealed the top differentially expressed exosomal miRNA, i.e. hsa-miR-139-5p (upregulation), had good performance to differentiate between MDD patients and controls. Tail vein injection of blood exosomes isolated from MDD patients into normal mice caused their depressive-like behaviors as determined by the forced swimming, tail suspension, and novelty suppressed feeding tests, and injection of blood exosomes isolated from healthy volunteers into unpredictable mild stress (CUMS)-treated mice alleviated their depressive-like behaviors. CUMS mice also showed significantly increased blood and brain levels of exosomal miR-139-5p. Furthermore, the depressive-like behaviors in CUMS-treated mice were rescued by intranasal injection of miR-139-5p antagomir, suggesting that increased exosomal miR-139-5p levels may mediate stress-induced depression-like behavior in mice. Both exosome treatment and miR-139-5p antagomir treatment increased hippocampal neurogenesis in the CUMS-treated mice, and treatment of exosome from MDD patients decreased hippocampal neurogenesis in the normal mice. The role of miR-139-5p in neurogenesis was validated by in vitro experiments, demonstrating that miR-139-5p is a negative regulator for neural stem cell proliferation and neuronal differentiation. Our findings together suggest that exosomes from patients with major depression caused depressive-like behaviors in mice with involvement of miR-139-5p-regulated neurogenesis. Therefore, exosomal miRNAs are promising targets for the diagnosis and treatment of MDD.
Figures
Similar articles
-
MiR-186-5p Dysregulation Leads to Depression-like Behavior by De-repressing SERPINF1 in Hippocampus.Neuroscience. 2021 Dec 15;479:48-59. doi: 10.1016/j.neuroscience.2021.10.005. Epub 2021 Oct 11. Neuroscience. 2021. PMID: 34648865
-
Clinical and preclinical evaluation of miR-144-5p as a key target for major depressive disorder.CNS Neurosci Ther. 2023 Nov;29(11):3598-3611. doi: 10.1111/cns.14291. Epub 2023 Jun 12. CNS Neurosci Ther. 2023. PMID: 37308778 Free PMC article.
-
MiR-129-5p prevents depressive-like behaviors by targeting MAPK1 to suppress inflammation.Exp Brain Res. 2021 Nov;239(11):3359-3370. doi: 10.1007/s00221-021-06203-8. Epub 2021 Sep 4. Exp Brain Res. 2021. PMID: 34482419
-
Synaptic plasticity and depression: the role of miRNAs dysregulation.Mol Biol Rep. 2022 Oct;49(10):9759-9765. doi: 10.1007/s11033-022-07461-7. Epub 2022 Apr 20. Mol Biol Rep. 2022. PMID: 35441941 Review.
-
Evidence for the Contribution of the miR-206/BDNF Pathway in the Pathophysiology of Depression.Int J Neuropsychopharmacol. 2024 Oct 1;27(10):pyae039. doi: 10.1093/ijnp/pyae039. Int J Neuropsychopharmacol. 2024. PMID: 39219169 Free PMC article. Review.
Cited by
-
miR-377-3p Regulates Hippocampal Neurogenesis via the Zfp462-Pbx1 Pathway and Mediates Anxiety-Like Behaviors in Prenatal Hypoxic Offspring.Mol Neurobiol. 2024 Apr;61(4):1920-1935. doi: 10.1007/s12035-023-03683-3. Epub 2023 Oct 11. Mol Neurobiol. 2024. PMID: 37817032
-
Multiomics and blood-based biomarkers of electroconvulsive therapy in severe and treatment-resistant depression: study protocol of the DetECT study.Eur Arch Psychiatry Clin Neurosci. 2024 Apr;274(3):673-684. doi: 10.1007/s00406-023-01647-1. Epub 2023 Aug 30. Eur Arch Psychiatry Clin Neurosci. 2024. PMID: 37644215 Free PMC article.
-
NK cell-derived exosomes carry miR-207 and alleviate depression-like symptoms in mice.J Neuroinflammation. 2020 Apr 22;17(1):126. doi: 10.1186/s12974-020-01787-4. J Neuroinflammation. 2020. PMID: 32321532 Free PMC article.
-
Running from stress: a perspective on the potential benefits of exercise-induced small extracellular vesicles for individuals with major depressive disorder.Front Mol Biosci. 2023 Jun 15;10:1154872. doi: 10.3389/fmolb.2023.1154872. eCollection 2023. Front Mol Biosci. 2023. PMID: 37398548 Free PMC article. Review.
-
Exosomes in brain diseases: Pathogenesis and therapeutic targets.MedComm (2020). 2023 Jun 11;4(3):e287. doi: 10.1002/mco2.287. eCollection 2023 Jun. MedComm (2020). 2023. PMID: 37313330 Free PMC article. Review.
References
-
- Varigonda AL, Jakubovski E, Taylor MJ, Freemantle N, Coughlin C, Bloch MH. Systematic review and meta-analysis: early treatment responses of selective serotonin reuptake inhibitors in pediatric major depressive disorder. J Am Acad Child Adolesc Psychiatry. 2015;54:557–64. doi: 10.1016/j.jaac.2015.05.004. - DOI - PubMed
-
- MacQueen G, Santaguida P, Keshavarz H, Jaworska N, Levine M, Beyene J. Systematic review of clinical practice guidelines for failed antidepressant treatment response in major depressive disorder, dysthymia, and subthreshold depression in adults. Can J Psychiatry. 2017;62:11–23. doi: 10.1177/0706743716664885. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

