Stem Cell Therapy for Diabetes: Beta Cells versus Pancreatic Progenitors
- PMID: 31979403
- PMCID: PMC7072676
- DOI: 10.3390/cells9020283
Stem Cell Therapy for Diabetes: Beta Cells versus Pancreatic Progenitors
Abstract
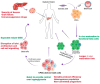
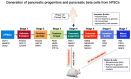
Diabetes mellitus (DM) is one of the most prevalent metabolic disorders. In order to replace the function of the destroyed pancreatic beta cells in diabetes, islet transplantation is the most widely practiced treatment. However, it has several limitations. As an alternative approach, human pluripotent stem cells (hPSCs) can provide an unlimited source of pancreatic cells that have the ability to secrete insulin in response to a high blood glucose level. However, the determination of the appropriate pancreatic lineage candidate for the purpose of cell therapy for the treatment of diabetes is still debated. While hPSC-derived beta cells are perceived as the ultimate candidate, their efficiency needs further improvement in order to obtain a sufficient number of glucose responsive beta cells for transplantation therapy. On the other hand, hPSC-derived pancreatic progenitors can be efficiently generated in vitro and can further mature into glucose responsive beta cells in vivo after transplantation. Herein, we discuss the advantages and predicted challenges associated with the use of each of the two pancreatic lineage products for diabetes cell therapy. Furthermore, we address the co-generation of functionally relevant islet cell subpopulations and structural properties contributing to the glucose responsiveness of beta cells, as well as the available encapsulation technology for these cells.
Keywords: hPSCs; hyperglycemia; insulin-secreting cells; pancreatic islets; transplantation; β-cell precursors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Lessons from Human Islet Transplantation Inform Stem Cell-Based Approaches in the Treatment of Diabetes.Front Endocrinol (Lausanne). 2021 Mar 11;12:636824. doi: 10.3389/fendo.2021.636824. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33776933 Free PMC article. Review.
-
Microporous scaffolds support assembly and differentiation of pancreatic progenitors into β-cell clusters.Acta Biomater. 2019 Sep 15;96:111-122. doi: 10.1016/j.actbio.2019.06.032. Epub 2019 Jun 25. Acta Biomater. 2019. PMID: 31247380 Free PMC article.
-
PDX1- /NKX6.1+ progenitors derived from human pluripotent stem cells as a novel source of insulin-secreting cells.Diabetes Metab Res Rev. 2021 Jul;37(5):e3400. doi: 10.1002/dmrr.3400. Epub 2020 Sep 2. Diabetes Metab Res Rev. 2021. PMID: 32857429
-
Islet-like organoids derived from human pluripotent stem cells efficiently function in the glucose responsiveness in vitro and in vivo.Sci Rep. 2016 Oct 12;6:35145. doi: 10.1038/srep35145. Sci Rep. 2016. PMID: 27731367 Free PMC article.
-
In Vivo Differentiation of Stem Cell-derived Human Pancreatic Progenitors to Treat Type 1 Diabetes.Stem Cell Rev Rep. 2020 Dec;16(6):1139-1155. doi: 10.1007/s12015-020-10018-5. Stem Cell Rev Rep. 2020. PMID: 32844324 Review.
Cited by
-
Dietary Magnesium Intake Affects the Vitamin D Effects on HOMA-β and Risk of Pancreatic β-Cell Dysfunction: A Cross-Sectional Study.Front Nutr. 2022 Mar 29;9:849747. doi: 10.3389/fnut.2022.849747. eCollection 2022. Front Nutr. 2022. PMID: 35425790 Free PMC article.
-
Current status of porcine islet xenotransplantation.Curr Opin Organ Transplant. 2020 Oct;25(5):449-456. doi: 10.1097/MOT.0000000000000794. Curr Opin Organ Transplant. 2020. PMID: 32773503 Free PMC article. Review.
-
Tailored generation of insulin producing cells from canine mesenchymal stem cells derived from bone marrow and adipose tissue.Sci Rep. 2021 Jun 11;11(1):12409. doi: 10.1038/s41598-021-91774-3. Sci Rep. 2021. PMID: 34117315 Free PMC article.
-
Long-Term Liraglutide Administration Induces Pancreas Neogenesis in Adult T2DM Mice.Cell Transplant. 2020 Jan-Dec;29:963689720927392. doi: 10.1177/0963689720927392. Cell Transplant. 2020. PMID: 32584149 Free PMC article.
-
Recent trends in stem cell-based therapies and applications of artificial intelligence in regenerative medicine.World J Stem Cells. 2021 Jun 26;13(6):521-541. doi: 10.4252/wjsc.v13.i6.521. World J Stem Cells. 2021. PMID: 34249226 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

