Recent Advancements in Non-Invasive Formulations for Protein Drug Delivery
- PMID: 31921395
- PMCID: PMC6944732
- DOI: 10.1016/j.csbj.2019.09.004
Recent Advancements in Non-Invasive Formulations for Protein Drug Delivery
Abstract
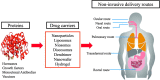
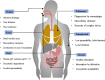
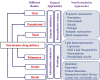
Advancements in biotechnology and protein engineering expand the availability of various therapeutic proteins including vaccines, antibodies, hormones, and growth factors. In addition, protein drugs hold many therapeutic advantages over small synthetic drugs in terms of high specificity and activity. This has led to further R&D investment in protein-based drug products and an increased number of drug approvals for therapeutic proteins. However, there are many biological and biopharmaceutical obstacles inherent to protein drugs including physicochemical and enzymatic destabilization, which limit their development and clinical application. Therefore, effective formulations of therapeutic proteins are needed to overcome the various physicochemical and biological barriers. In current medical practice, protein drugs are predominantly available in injectable formulations, which have disadvantages including pain, the possibility of infection, high cost, and low patient compliance. Consequently, non-invasive drug delivery systems for therapeutic proteins have gained great attention in the research and development of biomedicines. Therefore, this review covers the various formulation approaches to optimizing the delivery properties of protein drugs with an emphasis on improving bioavailability and patient compliance. It provides a comprehensive update on recent advancements in nanotechnologies with regard to non-invasive protein drug delivery systems, which is also categorized by the route of administrations including oral, nasal, transdermal, pulmonary, ocular, and rectal delivery systems.
Keywords: Drug delivery; Nanotechnology; Non-invasive; Non-parenteral; Protein.
© 2019 The Authors.
Conflict of interest statement
None.
Figures
Similar articles
-
Emerging delivery platforms for mucosal administration of biopharmaceuticals: a critical update on nasal, pulmonary and oral routes.Expert Opin Drug Deliv. 2017 Jan;14(1):23-36. doi: 10.1080/17425247.2016.1206074. Epub 2016 Jul 14. Expert Opin Drug Deliv. 2017. PMID: 27351299 Review.
-
An update on the application of physical technologies to enhance intradermal and transdermal drug delivery.Ther Deliv. 2012 Mar;3(3):339-55. doi: 10.4155/tde.12.1. Ther Deliv. 2012. PMID: 22833994 Review.
-
Nanotechnology for protein delivery: Overview and perspectives.J Control Release. 2016 Oct 28;240:24-37. doi: 10.1016/j.jconrel.2015.10.012. Epub 2015 Oct 13. J Control Release. 2016. PMID: 26458789 Free PMC article. Review.
-
Palatal mucosa as a route for systemic drug delivery: A review.J Control Release. 2011 Apr 10;151(1):2-9. doi: 10.1016/j.jconrel.2010.11.003. Epub 2010 Nov 6. J Control Release. 2011. PMID: 21059376 Review.
-
A Comprehensive Review of Challenges in Oral Drug Delivery Systems and Recent Advancements in Innovative Design Strategies.Curr Pharm Des. 2024 Oct 10. doi: 10.2174/0113816128338560240923073357. Online ahead of print. Curr Pharm Des. 2024. PMID: 39390835
Cited by
-
Re-Assessing PK/PD Issues for Oral Protein and Peptide Delivery.Pharmaceutics. 2021 Jul 2;13(7):1006. doi: 10.3390/pharmaceutics13071006. Pharmaceutics. 2021. PMID: 34371698 Free PMC article.
-
Influencing factors and drug application of iontophoresis in transdermal drug delivery: an overview of recent progress.Drug Deliv Transl Res. 2022 Jan;12(1):15-26. doi: 10.1007/s13346-021-00898-6. Epub 2021 Jan 23. Drug Deliv Transl Res. 2022. PMID: 33486687 Review.
-
Intravitreal long-term sustained ranibizumab delivery using injectable microgel-embedded hydrogel.Asian J Pharm Sci. 2024 Oct;19(5):100947. doi: 10.1016/j.ajps.2024.100947. Epub 2024 Aug 8. Asian J Pharm Sci. 2024. PMID: 39474125 Free PMC article.
-
Protein Nanoparticles: Uniting the Power of Proteins with Engineering Design Approaches.Adv Sci (Weinh). 2022 Mar;9(8):e2104012. doi: 10.1002/advs.202104012. Epub 2022 Jan 25. Adv Sci (Weinh). 2022. PMID: 35077010 Free PMC article. Review.
-
Modulation of Drug Release from Natural Polymer Matrices by Response Surface Methodology: in vitro and in vivo Evaluation.Drug Des Devel Ther. 2020 Dec 1;14:5325-5336. doi: 10.2147/DDDT.S279955. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 33293794 Free PMC article.
References
-
- Herrero E.P., Alonso M.J., Csaba N. Polymer-based oral peptide nanomedicines. Ther Deliv. 2012;3:657–668. - PubMed
-
- Sachdeva S., Lobo S., Goswami T. What is the future of noninvasive routes for protein- and peptide-based drugs? Ther Deliv. 2016;7:355–357. - PubMed
-
- U.S. Food & Drug Administration's Center for drug evaluation and research . New drug therapy approvals. 2019. Advancing health through innovation 2018.https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and... Available: Accessed 2019 Feb 03.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources