Commensal Bacteroidetes protect against Klebsiella pneumoniae colonization and transmission through IL-36 signalling
- PMID: 31907407
- PMCID: PMC7610889
- DOI: 10.1038/s41564-019-0640-1
Commensal Bacteroidetes protect against Klebsiella pneumoniae colonization and transmission through IL-36 signalling
Abstract
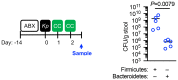
The microbiota primes immune defences but the identity of specific commensal microorganisms that protect against infection is unclear. Conversely, how pathogens compete with the microbiota to establish their host niche is also poorly understood. In the present study, we investigate the antagonism between the microbiota and Klebsiella pneumoniae during colonization and transmission. We discover that maturation of the microbiota drives the development of distinct immune defence programmes in the upper airways and intestine to limit K. pneumoniae colonization within these niches. Immune protection in the intestine depends on the development of Bacteroidetes, interleukin (IL)-36 signalling and macrophages. This effect of Bacteroidetes requires the polysaccharide utilization locus of their conserved commensal colonization factor. Conversely, in the upper airways, Proteobacteria prime immunity through IL-17A, but K. pneumoniae overcomes these defences through encapsulation to effectively colonize this site. Ultimately, we find that host-to-host spread of K. pneumoniae occurs principally from its intestinal reservoir, and that commensal-colonization-factor-producing Bacteroidetes are sufficient to prevent transmission between hosts through IL-36. Thus, our study provides mechanistic insight into when, where and how commensal Bacteroidetes protect against K. pneumoniae colonization and contagion, providing insight into how these protective microorganisms could be harnessed to confer population-level protection against K. pneumoniae infection.
Conflict of interest statement
The authors declare no competing interests.
Figures








Similar articles
-
Microbiota-mediated protection against antibiotic-resistant pathogens.Genes Immun. 2021 Oct;22(5-6):255-267. doi: 10.1038/s41435-021-00129-5. Epub 2021 May 4. Genes Immun. 2021. PMID: 33947987 Free PMC article. Review.
-
Animal Model To Study Klebsiella pneumoniae Gastrointestinal Colonization and Host-to-Host Transmission.Infect Immun. 2020 Oct 19;88(11):e00071-20. doi: 10.1128/IAI.00071-20. Print 2020 Oct 19. Infect Immun. 2020. PMID: 32839189 Free PMC article.
-
Distinct but Spatially Overlapping Intestinal Niches for Vancomycin-Resistant Enterococcus faecium and Carbapenem-Resistant Klebsiella pneumoniae.PLoS Pathog. 2015 Sep 3;11(9):e1005132. doi: 10.1371/journal.ppat.1005132. eCollection 2015 Sep. PLoS Pathog. 2015. PMID: 26334306 Free PMC article.
-
The microbiota protects against respiratory infection via GM-CSF signaling.Nat Commun. 2017 Nov 15;8(1):1512. doi: 10.1038/s41467-017-01803-x. Nat Commun. 2017. PMID: 29142211 Free PMC article.
-
Deciphering the gastrointestinal carriage of Klebsiella pneumoniae.Infect Immun. 2024 Sep 10;92(9):e0048223. doi: 10.1128/iai.00482-23. Epub 2024 Apr 10. Infect Immun. 2024. PMID: 38597634 Free PMC article. Review.
Cited by
-
Gut community structure as a risk factor for infection in Klebsiella pneumoniae-colonized patients.mSystems. 2024 Aug 20;9(8):e0078624. doi: 10.1128/msystems.00786-24. Epub 2024 Jul 8. mSystems. 2024. PMID: 38975759 Free PMC article.
-
Bibliometric analysis of intestinal microbiota and lung diseases.Front Cell Infect Microbiol. 2024 Feb 15;14:1347110. doi: 10.3389/fcimb.2024.1347110. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38426014 Free PMC article.
-
Mucin-degrading gut commensals isolated from healthy faecal donor suppress intestinal epithelial inflammation and regulate tight junction barrier function.Front Immunol. 2022 Oct 12;13:1021094. doi: 10.3389/fimmu.2022.1021094. eCollection 2022. Front Immunol. 2022. PMID: 36311778 Free PMC article.
-
Rujin Jiedu decoction protects against influenza virus infection by modulating gut microbiota.Heliyon. 2024 Jul 4;10(13):e34055. doi: 10.1016/j.heliyon.2024.e34055. eCollection 2024 Jul 15. Heliyon. 2024. PMID: 39071618 Free PMC article.
-
Microbiota-mediated protection against antibiotic-resistant pathogens.Genes Immun. 2021 Oct;22(5-6):255-267. doi: 10.1038/s41435-021-00129-5. Epub 2021 May 4. Genes Immun. 2021. PMID: 33947987 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

