Promoting tau secretion and propagation by hyperactive p300/CBP via autophagy-lysosomal pathway in tauopathy
- PMID: 31906970
- PMCID: PMC6945522
- DOI: 10.1186/s13024-019-0354-0
Promoting tau secretion and propagation by hyperactive p300/CBP via autophagy-lysosomal pathway in tauopathy
Abstract
Background: The trans-neuronal propagation of tau has been implicated in the progression of tau-mediated neurodegeneration. There is critical knowledge gap in understanding how tau is released and transmitted, and how that is dysregulated in diseases. Previously, we reported that lysine acetyltransferase p300/CBP acetylates tau and regulates its degradation and toxicity. However, whether p300/CBP is involved in regulation of tau secretion and propagation is unknown.
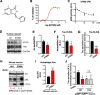
Method: We investigated the relationship between p300/CBP activity, the autophagy-lysosomal pathway (ALP) and tau secretion in mouse models of tauopathy and in cultured rodent and human neurons. Through a high-through-put compound screen, we identified a new p300 inhibitor that promotes autophagic flux and reduces tau secretion. Using fibril-induced tau spreading models in vitro and in vivo, we examined how p300/CBP regulates tau propagation.
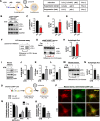
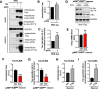
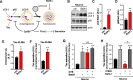
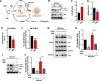
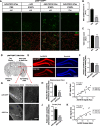
Results: Increased p300/CBP activity was associated with aberrant accumulation of ALP markers in a tau transgenic mouse model. p300/CBP hyperactivation blocked autophagic flux and increased tau secretion in neurons. Conversely, inhibiting p300/CBP promoted autophagic flux, reduced tau secretion, and reduced tau propagation in fibril-induced tau spreading models in vitro and in vivo.
Conclusions: We report that p300/CBP, a lysine acetyltransferase aberrantly activated in tauopathies, causes impairment in ALP, leading to excess tau secretion. This effect, together with increased intracellular tau accumulation, contributes to enhanced spreading of tau. Our findings suggest that inhibition of p300/CBP as a novel approach to correct ALP dysfunction and block disease progression in tauopathy.
Keywords: Autophagy-lysosomal pathway; Tau secretion; Tau spreading; Tauopathy; p300/CBP.
Conflict of interest statement
L. G. is a founder of Aeton Therapeutics.
Figures

Similar articles
-
Acetylation of tau inhibits its degradation and contributes to tauopathy.Neuron. 2010 Sep 23;67(6):953-66. doi: 10.1016/j.neuron.2010.08.044. Neuron. 2010. PMID: 20869593 Free PMC article.
-
Decrease of neuronal FKBP4/FKBP52 modulates perinuclear lysosomal positioning and MAPT/Tau behavior during MAPT/Tau-induced proteotoxic stress.Autophagy. 2021 Nov;17(11):3491-3510. doi: 10.1080/15548627.2021.1875611. Epub 2021 Jan 25. Autophagy. 2021. PMID: 33459145 Free PMC article.
-
Stimulation of synaptic activity promotes TFEB-mediated clearance of pathological MAPT/Tau in cellular and mouse models of tauopathies.Autophagy. 2023 Feb;19(2):660-677. doi: 10.1080/15548627.2022.2095791. Epub 2022 Jul 22. Autophagy. 2023. PMID: 35867714 Free PMC article.
-
Role of the endolysosomal pathway and exosome release in tau propagation.Neurochem Int. 2021 May;145:104988. doi: 10.1016/j.neuint.2021.104988. Epub 2021 Feb 11. Neurochem Int. 2021. PMID: 33582164 Review.
-
Autophagy and Tau Protein.Int J Mol Sci. 2021 Jul 12;22(14):7475. doi: 10.3390/ijms22147475. Int J Mol Sci. 2021. PMID: 34299093 Free PMC article. Review.
Cited by
-
Lysosome dysfunction as a cause of neurodegenerative diseases: Lessons from frontotemporal dementia and amyotrophic lateral sclerosis.Neurobiol Dis. 2021 Jul;154:105360. doi: 10.1016/j.nbd.2021.105360. Epub 2021 Mar 31. Neurobiol Dis. 2021. PMID: 33812000 Free PMC article. Review.
-
The Multiple Roles of Autophagy in Neural Function and Diseases.Neurosci Bull. 2024 Mar;40(3):363-382. doi: 10.1007/s12264-023-01120-y. Epub 2023 Oct 19. Neurosci Bull. 2024. PMID: 37856037 Free PMC article. Review.
-
Degradation and Transmission of Tau by Autophagic-Endolysosomal Networks and Potential Therapeutic Targets for Tauopathy.Front Mol Neurosci. 2020 Oct 16;13:586731. doi: 10.3389/fnmol.2020.586731. eCollection 2020. Front Mol Neurosci. 2020. PMID: 33177989 Free PMC article. Review.
-
Genome-Wide Association Analysis across Endophenotypes in Alzheimer's Disease: Main Effects and Disease Stage-Specific Interactions.Genes (Basel). 2023 Oct 27;14(11):2010. doi: 10.3390/genes14112010. Genes (Basel). 2023. PMID: 38002954 Free PMC article.
-
Identification of retinoblastoma binding protein 7 (Rbbp7) as a mediator against tau acetylation and subsequent neuronal loss in Alzheimer's disease and related tauopathies.Acta Neuropathol. 2021 Aug;142(2):279-294. doi: 10.1007/s00401-021-02323-1. Epub 2021 May 12. Acta Neuropathol. 2021. PMID: 33978814 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

