Laminin α2, α4, and α5 Chains Positively Regulate Migration and Survival of Oligodendrocyte Precursor Cells
- PMID: 31882770
- PMCID: PMC6934537
- DOI: 10.1038/s41598-019-56488-7
Laminin α2, α4, and α5 Chains Positively Regulate Migration and Survival of Oligodendrocyte Precursor Cells
Abstract
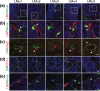
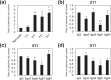
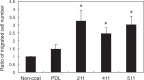
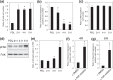
In the developing central nervous system (CNS), oligodendrocyte precursor cells (OPCs) migrate along blood vessels and are widely distributed in the CNS. Meanwhile, OPCs require survival factors from the extracellular microenvironment. In other tissues, laminins, heterotrimetric (αβγ) extracellular matrix proteins, promote cell migration and survival. However, the expression pattern and functions of laminins in OPC development remain poorly understood. In the present study, we first investigated the expression of laminin α chains, which bind to cell surface receptors such as integrins, in the postnatal murine brain. We found that laminin α1, α2, α4, and α5 chains were expressed around blood vessels and OPCs attached the laminin α chain-positive vessels. We then evaluated the effect of these laminins on OPCs activity using recombinant laminin E8s (LME8s) that are minimally active fragments of the laminin isoforms. OPCs attached on LM211E8, LM411E8, and LM511E8, containing laminin α2, α4, and α5 chains, respectively, through integrin β1. Further, these three LME8s promoted migration of OPCs, and OPC survival was prolonged on either LM411E8 or LM511E8 via the activation of focal adhesion kinase. Together, our findings suggest that laminins expressed surrounding blood vessels positively regulate migration and survival of OPCs through the integrin β1-FAK pathway.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Laminin isoforms and their integrin receptors in glioma cell migration and invasiveness: Evidence for a role of alpha5-laminin(s) and alpha3beta1 integrin.Exp Cell Res. 2007 Nov 1;313(18):3819-31. doi: 10.1016/j.yexcr.2007.07.038. Epub 2007 Aug 16. Exp Cell Res. 2007. PMID: 17888902
-
Laminin isoforms of lymph nodes and predominant role of alpha5-laminin(s) in adhesion and migration of blood lymphocytes.J Leukoc Biol. 2008 Sep;84(3):701-12. doi: 10.1189/jlb.0108048. Epub 2008 Jun 12. J Leukoc Biol. 2008. PMID: 18523231
-
α1- and α5-containing laminins regulate the development of bile ducts via β1 integrin signals.J Biol Chem. 2012 Aug 17;287(34):28586-97. doi: 10.1074/jbc.M112.350488. Epub 2012 Jul 3. J Biol Chem. 2012. PMID: 22761447 Free PMC article.
-
[Laminins in Metastatic Cancer].Mol Biol (Mosk). 2018 May-Jun;52(3):411-434. doi: 10.7868/S0026898418030059. Mol Biol (Mosk). 2018. PMID: 29989574 Review. Russian.
-
Expression and function of laminins in the embryonic and mature vasculature.Physiol Rev. 2005 Jul;85(3):979-1000. doi: 10.1152/physrev.00014.2004. Physiol Rev. 2005. PMID: 15987800 Review.
Cited by
-
Developmental Cues and Molecular Drivers in Myelinogenesis: Revisiting Early Life to Re-Evaluate the Integrity of CNS Myelin.Curr Issues Mol Biol. 2022 Jul 19;44(7):3208-3237. doi: 10.3390/cimb44070222. Curr Issues Mol Biol. 2022. PMID: 35877446 Free PMC article. Review.
-
Glutamate Signaling via the AMPAR Subunit GluR4 Regulates Oligodendrocyte Progenitor Cell Migration in the Developing Spinal Cord.J Neurosci. 2021 Jun 23;41(25):5353-5371. doi: 10.1523/JNEUROSCI.2562-20.2021. Epub 2021 May 11. J Neurosci. 2021. PMID: 33975920 Free PMC article.
-
Poly-L-ornithine blocks the inhibitory effects of fibronectin on oligodendrocyte differentiation and promotes myelin repair.Neural Regen Res. 2023 Apr;18(4):832-839. doi: 10.4103/1673-5374.353493. Neural Regen Res. 2023. PMID: 36204851 Free PMC article.
-
Crosstalk between Blood Vessels and Glia during the Central Nervous System Development.Life (Basel). 2022 Nov 1;12(11):1761. doi: 10.3390/life12111761. Life (Basel). 2022. PMID: 36362915 Free PMC article. Review.
-
The guanine nucleotide exchange factor Vav3 intervenes in the migration pathway of oligodendrocyte precursor cells on tenascin-C.Front Cell Dev Biol. 2022 Nov 30;10:1042403. doi: 10.3389/fcell.2022.1042403. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36531963 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

