A single-institution, randomized, pilot study evaluating the efficacy of gabapentin and methadone for patients undergoing chemoradiation for head and neck squamous cell cancer
- PMID: 31869451
- PMCID: PMC7069789
- DOI: 10.1002/cncr.32676
A single-institution, randomized, pilot study evaluating the efficacy of gabapentin and methadone for patients undergoing chemoradiation for head and neck squamous cell cancer
Erratum in
-
Erratum to "A single-institution, randomized, pilot study evaluating the efficacy of gabapentin and methadone for patients undergoing chemoradiation for head and neck squamous cell cancer".Cancer. 2022 Dec 1;128(23):4166. doi: 10.1002/cncr.34513. Epub 2022 Oct 17. Cancer. 2022. PMID: 36250257 No abstract available.
Abstract
Background: The objective of the current study was to compare the safety and efficacy between 2 analgesic regimens for patients with head and neck cancer (HNC) undergoing definitive chemoradiation (CRT).
Methods: The current study was a prospective, single-institution, 2-arm, randomized pilot study. Patients with American Joint Committee on Cancer seventh edition stage II to stage IV squamous cell carcinoma of the head and neck who were undergoing CRT were randomized to either arm 1, which entailed high-dose gabapentin (2700 mg daily) with the institutional standard of care (hydrocodone and/or acetaminophen progressing to fentanyl as needed), or arm 2, which comprised low-dose gabapentin (900 mg daily) with methadone. The primary endpoints were safety and toxicity. Secondary endpoints were pain, opioid requirement, and quality of life (QOL). Differences between the treatment arms at multiple time points were compared using a generalized linear mixed regression model with Sidak correction.
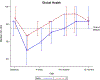
Results: A total of 60 patients (31 in arm 1 and 29 in arm 2) were enrolled from April 2015 to August 2017. There was no difference between the treatment arms with regard to adverse events or serious adverse events. Pain was not found to be different between the treatment arms. More patients in arm 1 did not require an opioid during treatment (42% vs 7%; P = .002). Patients in arm 2 experienced significantly better QOL outcomes across multiple domains, including overall health (P = .05), physical functioning (P = .04), role functioning (P = .01), and social functioning (P = .01).
Conclusions: High-dose prophylactic gabapentin increased the percentage of patients who required no opioid during treatment. Methadone may improve QOL compared with a regimen of short-acting opioids and fentanyl. However, pain was found to significantly worsen throughout treatment regardless of treatment arm, necessitating further studies to identify a more optimal regimen.
Keywords: European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30; Oral Mucositis Weekly Questionnaire adapted for head and neck cancer (OMWQ-HN); oral mucositis; patient-report outcomes; quality of life; version 3.0 (EORTC QLQ-C30).
© 2019 American Cancer Society.
Conflict of interest statement
Conflict of interest
All authors declare that they have no competing interests.
Figures





Similar articles
-
Randomized trial of standard pain control with or without gabapentin for pain related to radiation-induced mucositis in head and neck cancer.Auris Nasus Larynx. 2016 Dec;43(6):677-84. doi: 10.1016/j.anl.2016.02.012. Epub 2016 Mar 15. Auris Nasus Larynx. 2016. PMID: 26992271 Clinical Trial.
-
Efficacy of Prophylactic High-Dose Gabapentin and Venlafaxine on Reducing Oral Mucositis Pain Among Patients Treated With Chemoradiation for Head and Neck Cancer: A Single-Institution, Phase 2, Randomized Clinical Trial.Int J Radiat Oncol Biol Phys. 2023 Jul 15;116(4):797-806. doi: 10.1016/j.ijrobp.2023.01.047. Epub 2023 Feb 1. Int J Radiat Oncol Biol Phys. 2023. PMID: 36736633 Clinical Trial.
-
Randomized Phase 3, Double-Blind, Placebo-Controlled Study of Prophylactic Gabapentin for the Reduction of Oral Mucositis Pain During the Treatment of Oropharyngeal Squamous Cell Carcinoma.Int J Radiat Oncol Biol Phys. 2022 Mar 15;112(4):926-937. doi: 10.1016/j.ijrobp.2021.11.012. Epub 2021 Nov 20. Int J Radiat Oncol Biol Phys. 2022. PMID: 34808255 Clinical Trial.
-
Weekly Low-Dose Versus Three-Weekly High-Dose Cisplatin for Concurrent Chemoradiation in Locoregionally Advanced Non-Nasopharyngeal Head and Neck Cancer: A Systematic Review and Meta-Analysis of Aggregate Data.Oncologist. 2017 Sep;22(9):1056-1066. doi: 10.1634/theoncologist.2017-0015. Epub 2017 May 22. Oncologist. 2017. PMID: 28533474 Free PMC article. Review.
-
Opioids and the management of chronic severe pain in the elderly: consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization Step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone).Pain Pract. 2008 Jul-Aug;8(4):287-313. doi: 10.1111/j.1533-2500.2008.00204.x. Epub 2008 May 23. Pain Pract. 2008. PMID: 18503626
Cited by
-
Temporal Trends in Opioid Prescribing Patterns Among Oncologists in the Medicare Population.J Natl Cancer Inst. 2021 Mar 1;113(3):274-281. doi: 10.1093/jnci/djaa110. J Natl Cancer Inst. 2021. PMID: 32785685 Free PMC article.
-
Referral Patterns of Outpatient Palliative Care among the Head and Neck Cancer Population.Int Arch Otorhinolaryngol. 2022 Jan 27;26(4):e538-e547. doi: 10.1055/s-0041-1741436. eCollection 2022 Oct. Int Arch Otorhinolaryngol. 2022. PMID: 36405476 Free PMC article.
-
Severe Oral Mucositis After Intensity-Modulated Radiation Therapy for Head and Neck Cancer.JAMA Netw Open. 2023 Oct 2;6(10):e2337265. doi: 10.1001/jamanetworkopen.2023.37265. JAMA Netw Open. 2023. PMID: 37819659 Free PMC article.
-
Overcoming Barriers to Radiation Oncology Access in Low-Resource Settings in the United States.Adv Radiat Oncol. 2021 Sep 10;6(6):100802. doi: 10.1016/j.adro.2021.100802. eCollection 2021 Nov-Dec. Adv Radiat Oncol. 2021. PMID: 34693080 Free PMC article.
-
Interventions to improve quality of life in patients with head and neck cancers receiving radiation therapy: a scoping review.Support Care Cancer. 2023 Dec 16;32(1):31. doi: 10.1007/s00520-023-08197-1. Support Care Cancer. 2023. PMID: 38102525
References
-
- Society AAC. Cancer Facts & Figures 2018. 2018.
-
- Network NCC. Head and Neck Cancers Version 2.2018. https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. Accessed January 3, 2019.
-
- Vera-Llonch M, Oster G, Hagiwara M, Sonis S. Oral mucositis in patients undergoing radiation treatment for head and neck carcinoma. Cancer. 2006;106(2):329–336. - PubMed
-
- Elting LS, Cooksley CD, Chambers MS, Garden AS. Risk, outcomes, and costs of radiation-induced oral mucositis among patients with head-and-neck malignancies. Int J Radiat Oncol Biol Phys. 2007;68(4):1110–1120. - PubMed
-
- Trotti A Toxicity in head and neck cancer: a review of trends and issues. Int J Radiat Oncol Biol Phys. 2000;47(1):1–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

