Endothelial Cell Calcium Signaling during Barrier Function and Inflammation
- PMID: 31866349
- PMCID: PMC7074364
- DOI: 10.1016/j.ajpath.2019.11.004
Endothelial Cell Calcium Signaling during Barrier Function and Inflammation
Abstract
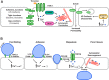
Calcium is an essential second messenger in endothelial cells and plays a pivotal role in regulating a number of physiologic processes, including cell migration, angiogenesis, barrier function, and inflammation. An increase in intracellular Ca2+ concentration can trigger a number of diverse signaling pathways under both physiologic and pathologic conditions. In this review, we discuss how calcium signaling pathways in endothelial cells play an essential role in affecting barrier function and facilitating inflammation. Inflammatory mediators, such as thrombin and histamine, increase intracellular calcium levels. This calcium influx causes adherens junction disassembly and cytoskeletal rearrangements to facilitate endothelial cell retraction and increased permeability. During inflammation endothelial cell calcium entry and the calcium-related signaling events also help facilitate several leukocyte-endothelial cell interactions, such as leukocyte rolling, adhesion, and ultimately transendothelial migration.
Copyright © 2020 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Role of Ca2+ signaling in the regulation of endothelial permeability.Vascul Pharmacol. 2002 Nov;39(4-5):173-85. doi: 10.1016/s1537-1891(03)00007-7. Vascul Pharmacol. 2002. PMID: 12747958 Review.
-
Ca2+ signaling, TRP channels, and endothelial permeability.Microcirculation. 2006 Dec;13(8):693-708. doi: 10.1080/10739680600930347. Microcirculation. 2006. PMID: 17085428 Review.
-
Protein kinase C-related kinase and ROCK are required for thrombin-induced endothelial cell permeability downstream from Galpha12/13 and Galpha11/q.J Biol Chem. 2008 Oct 31;283(44):29888-96. doi: 10.1074/jbc.M803880200. Epub 2008 Aug 19. J Biol Chem. 2008. PMID: 18713748 Free PMC article.
-
Ezrin/radixin/moesin proteins differentially regulate endothelial hyperpermeability after thrombin.Am J Physiol Lung Cell Mol Physiol. 2013 Aug 1;305(3):L240-55. doi: 10.1152/ajplung.00355.2012. Epub 2013 May 31. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 23729486 Free PMC article.
-
Endothelial microvesicles carrying Src-rich cargo impair adherens junction integrity and cytoskeleton homeostasis.Cardiovasc Res. 2020 Jul 1;116(8):1525-1538. doi: 10.1093/cvr/cvz238. Cardiovasc Res. 2020. PMID: 31504252 Free PMC article.
Cited by
-
Ecklonia cava Extract and Its Derivative Dieckol Promote Vasodilation by Modulating Calcium Signaling and PI3K/AKT/eNOS Pathway in In Vitro and In Vivo Models.Biomedicines. 2021 Apr 19;9(4):438. doi: 10.3390/biomedicines9040438. Biomedicines. 2021. PMID: 33921856 Free PMC article.
-
Activation of NMDA receptors in brain endothelial cells increases transcellular permeability.Fluids Barriers CNS. 2022 Sep 6;19(1):70. doi: 10.1186/s12987-022-00364-6. Fluids Barriers CNS. 2022. PMID: 36068542 Free PMC article.
-
Subchondral osteoclasts and osteoarthritis: new insights and potential therapeutic avenues.Acta Biochim Biophys Sin (Shanghai). 2024 Apr 25;56(4):499-512. doi: 10.3724/abbs.2024017. Acta Biochim Biophys Sin (Shanghai). 2024. PMID: 38439665 Free PMC article. Review.
-
Cross-Domain Text Mining of Pathophysiological Processes Associated with Diabetic Kidney Disease.Int J Mol Sci. 2024 Apr 19;25(8):4503. doi: 10.3390/ijms25084503. Int J Mol Sci. 2024. PMID: 38674089 Free PMC article.
-
Therapeutic Strategies for Angiogenesis Based on Endothelial Cell Epigenetics.J Cardiovasc Transl Res. 2024 Aug;17(4):816-827. doi: 10.1007/s12265-024-10485-y. Epub 2024 Jan 31. J Cardiovasc Transl Res. 2024. PMID: 38294628 Review.
References
-
- Clapham D.E. Calcium signaling. Cell. 2007;131:1047–1058. - PubMed
-
- Tiruppathi C., Minshall R.D., Paria B.C., Vogel S.M., Malik A.B. Role of Ca2+ signaling in the regulation of endothelial permeability. Vascul Pharmacol. 2002;39:173–185. - PubMed
-
- Berridge M.J., Bootman M.D., Roderick H.L. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. - PubMed
-
- Tran Q.K., Ohashi K., Watanabe H. Calcium signalling in endothelial cells. Cardiovasc Res. 2000;48:13–22. - PubMed
-
- Tiruppathi C., Ahmmed G.U., Vogel S.M., Malik A.B. Ca2+ signaling, TRP channels, and endothelial permeability. Microcirculation. 2006;13:693–708. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

