Microwave Assisted Synthesis, Characterization and Biological Activities of Ferrocenyl Chalcones and Their QSAR Analysis
- PMID: 31850307
- PMCID: PMC6901998
- DOI: 10.3389/fchem.2019.00814
Microwave Assisted Synthesis, Characterization and Biological Activities of Ferrocenyl Chalcones and Their QSAR Analysis
Abstract
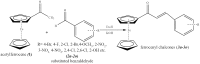
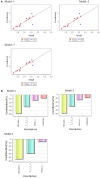
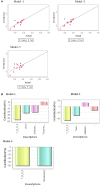
A new microwave method (MM) has been developed for the synthesis of a series of 16 substituted ferrocenyl chalcones using acetylferrocene (1) with different aldehydes (2a-2p) and comparing it with conventional method (CM). The synthesized compounds were characterized by various spectroscopic techniques viz IR, HR-MS, 1H NMR, and 13C NMR. The time required for completion of reaction in MM varied from 1 to 5 min as compared to CM which required 10-40 h. All the synthesized compounds were screened for antifungal activity against Sclerotium rolfsii and Alternaria solani. In vitro fungicidal activity revealed that compound 3o (ED50 = 23.24 mg L-1) was found to be most active against S. rolfsii. But in case of A. solani, compound 3c (ED50 = 29.9 mg L-1) showed highest activity. The nematicidal activity revealed that the compound 3b was more potent with LC50 values of 10.67, 7.30, and 4.55 ppm at 24, 48, and 72 h, respectively. 2D-Quantitative Structural Activity Relationship (2D-QSAR) analysis of these ferrocenyl chalcones was carried out by developing three different models namely Partial Least Squares (PLS, Model 1), Multiple Linear Regression (MLR, Model 2) and Principal Component Regression (PCR, Model 3). Statistical significance and predictive ability of these models were assessed by internal and external validation and also verified by leave one-out cross-validation. QSAR study revealed that MLR for S. rolfsii (r 2 = 0.999, q 2 = 0.996), PLS for A. solani (r 2 = 0.934, q 2 = 0.749) and PCR for M. incognita (r 2 = 0.878, q 2 = 0.772) were the best model. The physico-chemical parameters were calculated using VLife MDS 4.6 software. QSAR study could be employed for structure optimization to achieve better activity.
Keywords: Alternaria solani; Meloidogyne incognita; QSAR; Sclerotium rolfsii; antifungal activity; ferrocenyl chalcones; root-knot nematode.
Copyright © 2019 Yadav, Kaushik, Pankaj, Rana, Kamil, Khatri and Shakil.
Figures



Similar articles
-
Synthesis, Biological Evaluation, and QSAR Studies of 3-Iodochromone Derivatives as Potential Fungicides.Front Chem. 2021 Apr 30;9:636882. doi: 10.3389/fchem.2021.636882. eCollection 2021. Front Chem. 2021. PMID: 33996743 Free PMC article.
-
Microwave assisted synthesis, characterization and biological activities of ferrocenyl chalcones and their QSAR analysis: Part II.J Environ Sci Health B. 2021;56(1):82-97. doi: 10.1080/03601234.2020.1838828. Epub 2020 Nov 5. J Environ Sci Health B. 2021. PMID: 33150825
-
Bioefficacy evaluation of ferrocenyl chalcones against Meloidogyne incognita and Sclerotium rolfsii infestation in tomato.J Environ Sci Health B. 2022;57(3):192-200. doi: 10.1080/03601234.2022.2042154. Epub 2022 Feb 22. J Environ Sci Health B. 2022. PMID: 35193479
-
Green synthesis, structure-activity relationships, in silico molecular docking, and antifungal activities of novel prenylated chalcones.Front Chem. 2024 Apr 26;12:1389848. doi: 10.3389/fchem.2024.1389848. eCollection 2024. Front Chem. 2024. PMID: 38746019 Free PMC article.
-
Microwave synthesis and antifungal evaluations of some chalcones and their derived diaryl-cyclohexenones.J Environ Sci Health B. 2010 Aug;45(6):524-30. doi: 10.1080/03601234.2010.493482. J Environ Sci Health B. 2010. PMID: 20574873
Cited by
-
BiCl3-catalyzed green synthesis of 4-hydroxy-2-quinolone analogues under microwave irradiation.RSC Adv. 2023 Sep 21;13(40):28030-28041. doi: 10.1039/d3ra05289c. eCollection 2023 Sep 18. RSC Adv. 2023. PMID: 37746335 Free PMC article.
-
Synthesis, Biological Evaluation, and QSAR Studies of 3-Iodochromone Derivatives as Potential Fungicides.Front Chem. 2021 Apr 30;9:636882. doi: 10.3389/fchem.2021.636882. eCollection 2021. Front Chem. 2021. PMID: 33996743 Free PMC article.
-
Significance of Chalcone Scaffolds in Medicinal Chemistry.Top Curr Chem (Cham). 2024 Jun 27;382(3):22. doi: 10.1007/s41061-024-00468-7. Top Curr Chem (Cham). 2024. PMID: 38937401 Review.
References
-
- Abeysinghe S. (2009). Efficacy of combine use of biocontrol agents on control of Sclerotium rolfsii and Rhizoctonia solani of Capsicum annuum. Arch. Phytopathol. Plant Protect. 42, 221–227. 10.1080/03235400600999406 - DOI
-
- Alam S. (2004). Synthesis, antibacterial and antifungal activity of some derivatives of 2-phenyl-chromen-4-one. J. Chem. Sci. 116, 325–331. 10.1007/BF02711433 - DOI
-
- Arends I. W., Sheldon R. A., Wallau M., Schuchardt U. (1997). Oxidative transformations of organic compounds mediated by redox molecular sieves. Angew. Chem. Int. Ed. Engl. 36, 1144–1163. 10.1002/anie.199711441 - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

