Clueless forms dynamic, insulin-responsive bliss particles sensitive to stress
- PMID: 31837288
- PMCID: PMC7080587
- DOI: 10.1016/j.ydbio.2019.12.004
Clueless forms dynamic, insulin-responsive bliss particles sensitive to stress
Abstract
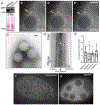
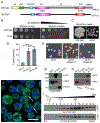
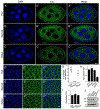
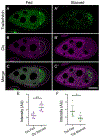
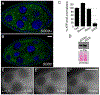
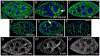
Drosophila Clueless (Clu) is a ribonucleoprotein that directly affects mitochondrial function. Loss of clu causes mitochondrial damage, and Clu associates with proteins on the mitochondrial outer membrane. Clu's subcellular pattern is diffuse throughout the cytoplasm, but Clu also forms large mitochondria-associated particles. Clu particles are reminiscent of ribonucleoprotein particles such as stress granules and processing bodies. Ribonucleoprotein particles play critical roles in the cell by regulating mRNAs spatially and temporally. Here, we show that Clu particles are unique, highly dynamic and rapidly disperse in response to stress in contrast to processing bodies and autophagosomes. In addition, Clu particle formation is dependent on diet as ovaries from starved females no longer contain Clu particles, and insulin signaling is necessary and sufficient for Clu particle formation. Oxidative stress also disperses particles. Since Clu particles are only present under optimal conditions, we have termed them "bliss particles". We also demonstrate that many aspects of Clu function are conserved in the yeast homolog Clu1p. These observations identify Clu particles as stress-sensitive cytoplasmic particles whose absence corresponds with altered cell stress and mitochondrial localization.
Keywords: Clueless; Drosophila; Insulin; Mitochondria; Ribonucleoprotein particle.
Published by Elsevier Inc.
Figures


Similar articles
-
Drosophila clueless is highly expressed in larval neuroblasts, affects mitochondrial localization and suppresses mitochondrial oxidative damage.PLoS One. 2013;8(1):e54283. doi: 10.1371/journal.pone.0054283. Epub 2013 Jan 16. PLoS One. 2013. PMID: 23342118 Free PMC article.
-
Clueless, a protein required for mitochondrial function, interacts with the PINK1-Parkin complex in Drosophila.Dis Model Mech. 2015 Jun;8(6):577-89. doi: 10.1242/dmm.019208. Epub 2015 Apr 20. Dis Model Mech. 2015. PMID: 26035866 Free PMC article.
-
Clueless, a conserved Drosophila gene required for mitochondrial subcellular localization, interacts genetically with parkin.Dis Model Mech. 2009 Sep-Oct;2(9-10):490-9. doi: 10.1242/dmm.002378. Epub 2009 Jul 28. Dis Model Mech. 2009. PMID: 19638420 Free PMC article.
-
Single particle imaging of mRNAs crossing the nuclear pore: Surfing on the edge.Bioessays. 2016 Aug;38(8):744-50. doi: 10.1002/bies.201600038. Epub 2016 Jun 8. Bioessays. 2016. PMID: 27276446 Review.
-
Nuclear export of mRNA.FEBS Lett. 2001 Jun 8;498(2-3):150-6. doi: 10.1016/s0014-5793(01)02482-6. FEBS Lett. 2001. PMID: 11412847 Review.
Cited by
-
Drosophila Clueless ribonucleoprotein particles display novel dynamics that rely on the availability of functional protein and polysome equilibrium.bioRxiv [Preprint]. 2024 Aug 22:2024.08.21.609023. doi: 10.1101/2024.08.21.609023. bioRxiv. 2024. PMID: 39229069 Free PMC article. Preprint.
-
Nutrient-dependent regulation of a stable intron modulates germline mitochondrial quality control.Nat Commun. 2024 Feb 10;15(1):1252. doi: 10.1038/s41467-024-45651-y. Nat Commun. 2024. PMID: 38341415 Free PMC article.
-
CLUH granules coordinate translation of mitochondrial proteins with mTORC1 signaling and mitophagy.EMBO J. 2020 May 4;39(9):e102731. doi: 10.15252/embj.2019102731. Epub 2020 Mar 9. EMBO J. 2020. PMID: 32149416 Free PMC article.
-
Loss of Drosophila Clueless differentially affects the mitochondrial proteome compared to loss of Sod2 and Pink1.Front Physiol. 2022 Oct 26;13:1004099. doi: 10.3389/fphys.2022.1004099. eCollection 2022. Front Physiol. 2022. PMID: 36388112 Free PMC article.
-
The interactome of CLUH reveals its association to SPAG5 and its co-translational proximity to mitochondrial proteins.BMC Biol. 2022 Jan 10;20(1):13. doi: 10.1186/s12915-021-01213-y. BMC Biol. 2022. PMID: 35012549 Free PMC article.
References
-
- Barbee SA, Estes PS, Cziko AM, Hillebrand J, Luedeman RA, Coller JM, … Ramaswami M (2006). Staufen- and FMRP-containing neuronal RNPs are structurally and functionally related to somatic P bodies. Neuron, 52(6), 997–1009. doi:S0896-6273(06)00827-0 [pii] 10.1016/j.neuron.2006.10.028 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

