A metastable subproteome underlies inclusion formation in muscle proteinopathies
- PMID: 31796104
- PMCID: PMC6891963
- DOI: 10.1186/s40478-019-0853-9
A metastable subproteome underlies inclusion formation in muscle proteinopathies
Abstract
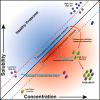
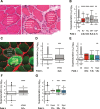
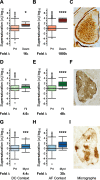
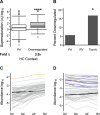
Protein aggregation is a pathological feature of neurodegenerative disorders. We previously demonstrated that protein inclusions in the brain are composed of supersaturated proteins, which are abundant and aggregation-prone, and form a metastable subproteome. It is not yet clear, however, whether this phenomenon is also associated with non-neuronal protein conformational disorders. To respond to this question, we analyzed proteomic datasets from biopsies of patients with genetic and acquired protein aggregate myopathy (PAM) by quantifying the changes in composition, concentration and aggregation propensity of proteins in the fibers containing inclusions and those surrounding them. We found that a metastable subproteome is present in skeletal muscle from healthy patients. The expression of this subproteome escalate as proteomic samples are taken more proximal to the pathologic inclusion, eventually exceeding its solubility limits and aggregating. While most supersaturated proteins decrease or maintain steady abundance across healthy fibers and inclusion-containing fibers, proteins within the metastable subproteome rise in abundance, suggesting that they escape regulation. Taken together, our results show in the context of a human conformational disorder that the supersaturation of a metastable subproteome underlies widespread aggregation and correlates with the histopathological state of the tissue.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Involvement of clusterin and the aggresome in abnormal protein deposits in myofibrillar myopathies and inclusion body myositis.Brain Pathol. 2005 Apr;15(2):101-8. doi: 10.1111/j.1750-3639.2005.tb00504.x. Brain Pathol. 2005. PMID: 15912881 Free PMC article.
-
Difference in expression of phosphorylated tau epitopes between sporadic inclusion-body myositis and hereditary inclusion-body myopathies.J Neuropathol Exp Neurol. 1996 Jul;55(7):774-86. doi: 10.1097/00005072-199607000-00003. J Neuropathol Exp Neurol. 1996. PMID: 8965093
-
Supersaturation is a major driving force for protein aggregation in neurodegenerative diseases.Trends Pharmacol Sci. 2015 Feb;36(2):72-7. doi: 10.1016/j.tips.2014.12.004. Epub 2015 Jan 27. Trends Pharmacol Sci. 2015. PMID: 25636813 Free PMC article. Review.
-
Amyloid-beta42 is preferentially accumulated in muscle fibers of patients with sporadic inclusion-body myositis.Acta Neuropathol. 2009 May;117(5):569-74. doi: 10.1007/s00401-009-0511-6. Epub 2009 Mar 12. Acta Neuropathol. 2009. PMID: 19280202
-
Inclusion body myositis: a degenerative muscle disease associated with intra-muscle fiber multi-protein aggregates, proteasome inhibition, endoplasmic reticulum stress and decreased lysosomal degradation.Brain Pathol. 2009 Jul;19(3):493-506. doi: 10.1111/j.1750-3639.2009.00290.x. Brain Pathol. 2009. PMID: 19563541 Free PMC article. Review.
Cited by
-
Mechanisms and pathology of protein misfolding and aggregation.Nat Rev Mol Cell Biol. 2023 Dec;24(12):912-933. doi: 10.1038/s41580-023-00647-2. Epub 2023 Sep 8. Nat Rev Mol Cell Biol. 2023. PMID: 37684425 Review.
-
Transgenic sensors reveal compartment-specific effects of aggregation-prone proteins on subcellular proteostasis during aging.Cell Rep Methods. 2024 Oct 21;4(10):100875. doi: 10.1016/j.crmeth.2024.100875. Epub 2024 Oct 8. Cell Rep Methods. 2024. PMID: 39383859 Free PMC article.
-
Targeting DNA topoisomerases or checkpoint kinases results in an overload of chaperone systems, triggering aggregation of a metastable subproteome.Elife. 2022 Feb 24;11:e70726. doi: 10.7554/eLife.70726. Elife. 2022. PMID: 35200138 Free PMC article.
-
Protein folding in vitro and in the cell: From a solitary journey to a team effort.Biophys Chem. 2022 Aug;287:106821. doi: 10.1016/j.bpc.2022.106821. Epub 2022 Apr 29. Biophys Chem. 2022. PMID: 35667131 Free PMC article. Review.
-
Bi-allelic variants of FILIP1 cause congenital myopathy, dysmorphism and neurological defects.Brain. 2023 Oct 3;146(10):4200-4216. doi: 10.1093/brain/awad152. Brain. 2023. PMID: 37163662 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

