Thawed Mesenchymal Stem Cell Product Shows Comparable Immunomodulatory Potency to Cultured Cells In Vitro and in Polymicrobial Septic Animals
- PMID: 31792313
- PMCID: PMC6889371
- DOI: 10.1038/s41598-019-54462-x
Thawed Mesenchymal Stem Cell Product Shows Comparable Immunomodulatory Potency to Cultured Cells In Vitro and in Polymicrobial Septic Animals
Abstract
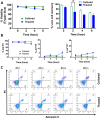
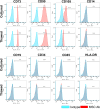
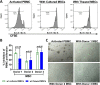
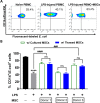
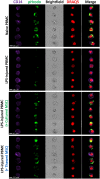
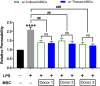
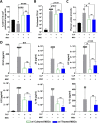
Mesenchymal stem cells (MSCs) have been shown to exert immunomodulatory effects in both acute and chronic diseases. In acute inflammatory conditions like sepsis, cell therapy must be administered within hours of diagnosis, requiring "off-the-shelf" cryopreserved allogeneic cell products. However, their immunomodulatory potency, particularly in abilities to modulate innate immune cells, has not been well documented. Herein we compared the stabilities and functionalities of cultured versus thawed, donor-matched MSCs in modulating immune responses in vitro and in vivo. Cultured and thawed MSCs exhibited similar surface marker profiles and viabilities at 0 hr; however, thawed MSCs exhibited higher levels of apoptotic cells beyond 4 hrs. In vitro potency assays showed no significant difference between the abilities of both MSCs (donor-matched) to suppress proliferation of activated T cells, enhance phagocytosis of monocytes, and restore endothelial permeability after injury. Most importantly, in animals with polymicrobial sepsis, both MSCs significantly improved the phagocytic ability of peritoneal lavage cells, and reduced plasma levels of lactate and selected inflammatory cytokines without significant difference between groups. These results show comparable in vitro and in vivo immunomodulatory efficacy of thawed and fresh MSC products, providing further evidence for the utility of a cryopreserved MSC product for acute inflammatory diseases.
Conflict of interest statement
The funding institution had no role in the conception, design or conduct of the study, data collection or analysis, interpretation or presentation of the data, or preparation, review or approval of the manuscript. We also like to declare the following conflicts of interest: D.J.S. holds a patent for MSC therapy for the treatment of acute lung injury, and S.H.J.M. has received personal fees from Northern Therapeutics that are outside of this submitted work. The remaining authors have disclosed that they do not have any conflicts of interest.
Figures
Similar articles
-
Cryopreserved Mesenchymal Stromal Cells Are Susceptible to T-Cell Mediated Apoptosis Which Is Partly Rescued by IFNγ Licensing.Stem Cells. 2016 Sep;34(9):2429-42. doi: 10.1002/stem.2415. Epub 2016 Jul 4. Stem Cells. 2016. PMID: 27299362 Free PMC article.
-
Do cryopreserved mesenchymal stromal cells display impaired immunomodulatory and therapeutic properties?Stem Cells. 2014 Sep;32(9):2430-42. doi: 10.1002/stem.1729. Stem Cells. 2014. PMID: 24805247 Free PMC article.
-
Trained murine mesenchymal stem cells have anti-inflammatory effect on macrophages, but defective regulation on T-cell proliferation.FASEB J. 2019 Mar;33(3):4203-4211. doi: 10.1096/fj.201801845R. Epub 2018 Dec 6. FASEB J. 2019. PMID: 30521384 Free PMC article.
-
Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of Action of Living, Apoptotic, and Dead MSCs.Front Immunol. 2019 Jun 4;10:1191. doi: 10.3389/fimmu.2019.01191. eCollection 2019. Front Immunol. 2019. PMID: 31214172 Free PMC article. Review.
-
Cryopreserved or Fresh Mesenchymal Stromal Cells: Only a Matter of Taste or Key to Unleash the Full Clinical Potential of MSC Therapy?Adv Exp Med Biol. 2016;951:77-98. doi: 10.1007/978-3-319-45457-3_7. Adv Exp Med Biol. 2016. PMID: 27837556 Review.
Cited by
-
Study of the Effect of Wild-Type and Transiently Expressing CXCR4 and IL-10 Mesenchymal Stromal Cells in a Mouse Model of Peritonitis.Int J Mol Sci. 2023 Dec 30;25(1):520. doi: 10.3390/ijms25010520. Int J Mol Sci. 2023. PMID: 38203690 Free PMC article.
-
Influence of Hypothermic Storage Fluids on Mesenchymal Stem Cell Stability: A Comprehensive Review and Personal Experience.Cells. 2021 Apr 28;10(5):1043. doi: 10.3390/cells10051043. Cells. 2021. PMID: 33925059 Free PMC article. Review.
-
Comparative Bioactivity Analysis for Off-the-Shelf and Culture-Rescued Umbilical Cord-Derived Mesenchymal Stem/Stromal Cells in a Xeno- and Serum-Free Culture System.Cell Transplant. 2021 Jan-Dec;30:9636897211039441. doi: 10.1177/09636897211039441. Cell Transplant. 2021. PMID: 34538123 Free PMC article.
-
Transplantation of olfactory mucosa mesenchymal stromal cells repairs spinal cord injury by inducing microglial polarization.Spinal Cord. 2024 Aug;62(8):429-439. doi: 10.1038/s41393-024-01004-6. Epub 2024 Jun 7. Spinal Cord. 2024. PMID: 38849489
-
Thawed cryopreserved synovial mesenchymal stem cells show comparable effects to cultured cells in the inhibition of osteoarthritis progression in rats.Sci Rep. 2021 May 6;11(1):9683. doi: 10.1038/s41598-021-89239-8. Sci Rep. 2021. PMID: 33958682 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

