Oncolytic Adenovirus-A Nova for Gene-Targeted Oncolytic Viral Therapy in HCC
- PMID: 31781493
- PMCID: PMC6857090
- DOI: 10.3389/fonc.2019.01182
Oncolytic Adenovirus-A Nova for Gene-Targeted Oncolytic Viral Therapy in HCC
Erratum in
-
Corrigendum: Oncolytic Adenovirus-A Nova for Gene-Targeted Oncolytic Viral Therapy in HCC.Front Oncol. 2020 May 5;10:701. doi: 10.3389/fonc.2020.00701. eCollection 2020. Front Oncol. 2020. PMID: 32432043 Free PMC article.
Abstract
Hepatocellular carcinoma (HCC) is one of the most frequent cancers worldwide, particularly in China. Despite the development of HCC treatment strategies, the survival rate remains unpleasant. Gene-targeted oncolytic viral therapy (GTOVT) is an emerging treatment modality-a kind of cancer-targeted therapy-which creates viral vectors armed with anti-cancer genes. The adenovirus is a promising agent for GAOVT due to its many advantages. In spite of the oncolytic adenovirus itself, the host immune response is the determining factor for the anti-cancer efficacy. In this review, we have summarized recent developments in oncolytic adenovirus engineering and the development of novel therapeutic genes utilized in HCC treatment. Furthermore, the diversified roles the immune response plays in oncolytic adenovirus therapy and recent attempts to modulate immune responses to enhance the anti-cancer efficacy of oncolytic adenovirus have been discussed.
Keywords: HCC; adenovirus; gene-targeted oncolytic viral therapy; immunotherapy; virus engineering.
Copyright © 2019 Abudoureyimu, Lai, Tian, Wang, Wang and Chu.
Figures
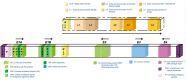
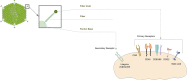

Similar articles
-
A novel Golgi protein (GOLPH2)-regulated oncolytic adenovirus exhibits potent antitumor efficacy in hepatocellular carcinoma.Oncotarget. 2015 May 30;6(15):13564-78. doi: 10.18632/oncotarget.3769. Oncotarget. 2015. PMID: 25980438 Free PMC article.
-
Corrigendum: Oncolytic Adenovirus-A Nova for Gene-Targeted Oncolytic Viral Therapy in HCC.Front Oncol. 2020 May 5;10:701. doi: 10.3389/fonc.2020.00701. eCollection 2020. Front Oncol. 2020. PMID: 32432043 Free PMC article.
-
Adenovirus and Immunotherapy: Advancing Cancer Treatment by Combination.Cancers (Basel). 2020 May 21;12(5):1295. doi: 10.3390/cancers12051295. Cancers (Basel). 2020. PMID: 32455560 Free PMC article. Review.
-
Mesenchymal Stem Cell-Mediated Delivery of an Oncolytic Adenovirus Enhances Antitumor Efficacy in Hepatocellular Carcinoma.Cancer Res. 2019 Sep 1;79(17):4503-4514. doi: 10.1158/0008-5472.CAN-18-3900. Epub 2019 Jul 9. Cancer Res. 2019. PMID: 31289131
-
Oncolytic Adenovirus: Prospects for Cancer Immunotherapy.Front Microbiol. 2021 Jul 21;12:707290. doi: 10.3389/fmicb.2021.707290. eCollection 2021. Front Microbiol. 2021. PMID: 34367111 Free PMC article. Review.
Cited by
-
From regulation to deregulation of p53 in hematologic malignancies: implications for diagnosis, prognosis and therapy.Biomark Res. 2024 Nov 14;12(1):137. doi: 10.1186/s40364-024-00676-9. Biomark Res. 2024. PMID: 39538363 Free PMC article. Review.
-
Peptide-Based Vaccines for Hepatocellular Carcinoma: A Review of Recent Advances.J Hepatocell Carcinoma. 2021 Sep 2;8:1035-1054. doi: 10.2147/JHC.S291558. eCollection 2021. J Hepatocell Carcinoma. 2021. PMID: 34513746 Free PMC article. Review.
-
Evolving Horizons: Adenovirus Vectors' Timeless Influence on Cancer, Gene Therapy and Vaccines.Viruses. 2023 Dec 3;15(12):2378. doi: 10.3390/v15122378. Viruses. 2023. PMID: 38140619 Free PMC article. Review.
-
Polymer Coated Oncolytic Adenovirus to Selectively Target Hepatocellular Carcinoma Cells.Pharmaceutics. 2021 Jun 24;13(7):949. doi: 10.3390/pharmaceutics13070949. Pharmaceutics. 2021. PMID: 34202714 Free PMC article.
-
Virus against virus: strategies for using adenovirus vectors in the treatment of HPV-induced cervical cancer.Acta Pharmacol Sin. 2021 Dec;42(12):1981-1990. doi: 10.1038/s41401-021-00616-5. Epub 2021 Feb 25. Acta Pharmacol Sin. 2021. PMID: 33633364 Free PMC article. Review.
References
-
- De Pace N. Sulla scomparsa di un enorme cancro vegetante del callo dell'utero senza cura chirurgica. Ginecologia. (1912) 1912:82–8.
Publication types
LinkOut - more resources
Full Text Sources

