Adipose-Derived Mesenchymal Stem Cells Enhance Ovarian Cancer Growth and Metastasis by Increasing Thymosin Beta 4X-Linked Expression
- PMID: 31781249
- PMCID: PMC6855023
- DOI: 10.1155/2019/9037197
Adipose-Derived Mesenchymal Stem Cells Enhance Ovarian Cancer Growth and Metastasis by Increasing Thymosin Beta 4X-Linked Expression
Abstract
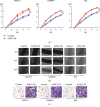
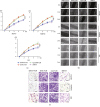
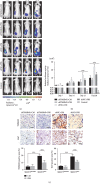
As shown in our previous studies, growth and metastasis of ovarian cancer can be regulated by adipose-derived mesenchymal stem cells (ADSCs). However, the underlying mechanism has not yet been revealed. In this study, a proteomics analysis was performed to compare protein expression treated with and without ADSCs in ovarian cancer cells. Protein levels were altered in ovarian cancer cells due to the treatment of ADSCs. Thymosin beta 4 X-linked (TMSB4X) levels changed dramatically, and this protein was identified as one of the most important candidate molecules contributing to the tumour-promoting effects of ADSCs. Compared with the cells that are cultured in the normal growth medium, the TMSB4X levels cultured in ADSC-conditioned medium increased significantly in ovarian cancer cells. Furthermore, the growth and invasion of cancer cells were decreased, even in the ADSC-conditioned medium treatment group (P < 0.05), by the inhibition of TMSB4X. As shown in the bioluminescence images captured in vivo, increased ovarian cancer's growth and metastasis, along with elevated TMSB4X expression, were observed in the group of ADSC-conditioned medium, and the tumour-promoting effect of ADSCs was attenuated by the inhibition of TMSB4X. Based on our findings, increased TMSB4X expression may play a role in accelerating the ADSC-mediated proliferation, invasion, and migration of ovarian cancers.
Copyright © 2019 Yijing Chu et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

Similar articles
-
Exosomes from Human Omental Adipose-Derived Mesenchymal Stem Cells Secreted into Ascites Promote Peritoneal Metastasis of Epithelial Ovarian Cancer.Cells. 2022 Oct 27;11(21):3392. doi: 10.3390/cells11213392. Cells. 2022. PMID: 36359787 Free PMC article.
-
Adipose-derived mesenchymal stem cells induced PAX8 promotes ovarian cancer cell growth by stabilizing TAZ protein.J Cell Mol Med. 2021 May;25(9):4434-4443. doi: 10.1111/jcmm.16511. Epub 2021 Apr 8. J Cell Mol Med. 2021. PMID: 33830648 Free PMC article.
-
Adipose-derived mesenchymal stem cells promote cell proliferation and invasion of epithelial ovarian cancer.Exp Cell Res. 2015 Sep 10;337(1):16-27. doi: 10.1016/j.yexcr.2015.07.020. Epub 2015 Jul 21. Exp Cell Res. 2015. PMID: 26209607
-
Adipose-derived mesenchymal stem cells promote osteosarcoma proliferation and metastasis by activating the STAT3 pathway.Oncotarget. 2017 Apr 4;8(14):23803-23816. doi: 10.18632/oncotarget.15866. Oncotarget. 2017. PMID: 28423603 Free PMC article.
-
The Basic Mechanism of Hair Growth Stimulation by Adipose-derived Stem Cells and Their Secretory Factors.Curr Stem Cell Res Ther. 2017;12(7):535-543. doi: 10.2174/1574888X12666170829161058. Curr Stem Cell Res Ther. 2017. PMID: 28875863 Review.
Cited by
-
Adipose-derived stem cells in ovarian cancer progression, metastasis, and chemoresistance.Exp Biol Med (Maywood). 2021 Aug;246(16):1810-1815. doi: 10.1177/15353702211023846. Epub 2021 Jul 6. Exp Biol Med (Maywood). 2021. PMID: 34229470 Free PMC article. Review.
-
A Transcriptomic Analysis of Head and Neck Squamous Cell Carcinomas for Prognostic Indications.J Pers Med. 2021 Aug 11;11(8):782. doi: 10.3390/jpm11080782. J Pers Med. 2021. PMID: 34442426 Free PMC article.
-
Omentum provides a special cell microenvironment for ovarian cancer.Cancer Rep (Hoboken). 2023 Oct;6(10):e1858. doi: 10.1002/cnr2.1858. Epub 2023 Aug 21. Cancer Rep (Hoboken). 2023. PMID: 37605299 Free PMC article. Review.
-
Circular RNA PIP5K1A (circPIP5K1A) accelerates endometriosis progression by regulating the miR-153-3p/Thymosin Beta-4 X-Linked (TMSB4X) pathway.Bioengineered. 2021 Dec;12(1):7104-7118. doi: 10.1080/21655979.2021.1978618. Bioengineered. 2021. PMID: 34546850 Free PMC article.
-
Potential Consequences of the Use of Adipose-Derived Stem Cells in the Treatment of Hepatocellular Carcinoma.Int J Mol Sci. 2024 Jul 17;25(14):7806. doi: 10.3390/ijms25147806. Int J Mol Sci. 2024. PMID: 39063048 Free PMC article. Review.
References
-
- Wu S., Zheng Q., Xing X., et al. Matrix stiffness-upregulated LOXL2 promotes fibronectin production, MMP9 and CXCL12 expression and BMDCs recruitment to assist pre-metastatic niche formation. Journal of Experimental & Clinical Cancer Research. 2018;37(1):p. 99. doi: 10.1186/s13046-018-0761-z. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

