Interleukin-1β Mediates Arterial Thrombus Formation via NET-Associated Tissue Factor
- PMID: 31779200
- PMCID: PMC6947515
- DOI: 10.3390/jcm8122072
Interleukin-1β Mediates Arterial Thrombus Formation via NET-Associated Tissue Factor
Abstract
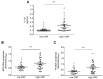
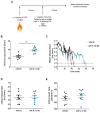
CANTOS reported reduced secondary atherothrombotic events in patients with residual inflammatory risk treated with the inhibitory anti-IL-1β antibody, Canakinumab. Yet, mechanisms that underlie this benefit remain elusive. Recent work has implicated formation of neutrophil extracellular traps (NETosis) in arterial thrombosis. Hence, the present study explored the potential link between IL-1β, NETs, and tissue factor (TF)-the key trigger of the coagulation cascade-in atherothrombosis. To this end, ST-elevation myocardial infarction (STEMI) patients from the Swiss multicenter trial SPUM-ACS were retrospectively and randomly selected based on their CRP levels. In particular, 33 patients with STEMI and high C-reactive protein (CRP) levels (≥ 10 mg/L) and, 33 with STEMI and low CRP levels (≤ 4 mg/L) were investigated. High CRP patients displayed elevated circulating IL-1β, NETosis, and NET-associated TF plasma levels compared with low CRP ones. Additionally, analysis of patients stratified by circulating IL-1β levels yielded similar results. Moreover, NETosis and NET-associated TF plasma levels correlated positively in the whole population. In addition to the above, translational research experiments provided mechanistic confirmation for the clinical data identifying IL-1β as the initial trigger for the release of the pro-coagulant, NET-associated TF. In conclusion, blunted TF presentation by activated neutrophils undergoing NETosis may provide a mechanistic explanation to reduced secondary atherothrombotic events as observed in canakinumab-treated patients in CANTOS.
Keywords: Canakinumab; IL-1β; arterial thrombosis; neutrophil extracellular traps; tissue factor.
Conflict of interest statement
T.F.L. and C.M.M. have been member of the Canakinumab advisory board of Novartis and have received honoraria as well as educational grants to the institution. P.L. is an unpaid consultant to, or involved in clinical trials for Amgen, AstraZeneca, Esperion Therapeutics, Ionis Pharmaceuticals, Kowa Pharmaceuticals, Novartis, Pfizer, Sanofi-Regeneron, and XBiotech, Inc. PL is a member of scientific advisory board for Amgen, Corvidia Therapeutics, DalCor Pharmaceuticals, IFM Therapeutics, Kowa Pharmaceuticals, Olatec Therapeutics, Medimmune, Novartis, and XBiotech, Inc. Libby’s laboratory has received research funding in the last 2 years from Novartis. All other authors declare no conflict of interest.
Figures



Similar articles
-
Interleukin-1β inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS).Am Heart J. 2011 Oct;162(4):597-605. doi: 10.1016/j.ahj.2011.06.012. Epub 2011 Sep 14. Am Heart J. 2011. PMID: 21982649 Clinical Trial.
-
Residual inflammatory risk associated with interleukin-18 and interleukin-6 after successful interleukin-1β inhibition with canakinumab: further rationale for the development of targeted anti-cytokine therapies for the treatment of atherothrombosis.Eur Heart J. 2020 Jun 14;41(23):2153-2163. doi: 10.1093/eurheartj/ehz542. Eur Heart J. 2020. PMID: 31504417 Clinical Trial.
-
Closing the loop on inflammation and atherothrombosis: why perform the CIRT and CANTOS trials?Trans Am Clin Climatol Assoc. 2013;124:174-90. Trans Am Clin Climatol Assoc. 2013. PMID: 23874021 Free PMC article.
-
Clinician's Guide to Reducing Inflammation to Reduce Atherothrombotic Risk: JACC Review Topic of the Week.J Am Coll Cardiol. 2018 Dec 25;72(25):3320-3331. doi: 10.1016/j.jacc.2018.06.082. Epub 2018 Nov 8. J Am Coll Cardiol. 2018. PMID: 30415883 Review.
-
NET-(works) in arterial and venous thrombo-occlusive diseases.Front Cardiovasc Med. 2023 May 22;10:1155512. doi: 10.3389/fcvm.2023.1155512. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37283578 Free PMC article. Review.
Cited by
-
Anakinra for severe forms of COVID-19: a cohort study.Lancet Rheumatol. 2020 Jul;2(7):e393-e400. doi: 10.1016/S2665-9913(20)30164-8. Epub 2020 May 29. Lancet Rheumatol. 2020. PMID: 32835245 Free PMC article.
-
Editorial Commentary: Top Five Stories of the Cellular Landscape and Therapies of Atherosclerosis: Current Knowledge and Future Perspectives.Curr Med Sci. 2024 Feb;44(1):241-243. doi: 10.1007/s11596-023-2825-3. Curr Med Sci. 2024. PMID: 38277018 No abstract available.
-
Philadelphia chromosome-negative myeloproliferative chronic neoplasms: is clonal hematopoiesis the main determinant of autoimmune and cardio-vascular manifestations?Front Med (Lausanne). 2023 Oct 17;10:1254868. doi: 10.3389/fmed.2023.1254868. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37915324 Free PMC article. Review.
-
Immune Modulation as a Therapeutic Option During the SARS-CoV-2 Outbreak: The Case for Antimalarial Aminoquinolines.Front Immunol. 2020 Aug 28;11:2159. doi: 10.3389/fimmu.2020.02159. eCollection 2020. Front Immunol. 2020. PMID: 32983179 Free PMC article. Review.
-
Neutrophils and Neutrophil Extracellular Traps Drive Necroinflammation in COVID-19.Cells. 2020 Jun 2;9(6):1383. doi: 10.3390/cells9061383. Cells. 2020. PMID: 32498376 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

