Distribution pattern and molecular signature of cholinergic tuft cells in human gastro-intestinal and pancreatic-biliary tract
- PMID: 31767912
- PMCID: PMC6877571
- DOI: 10.1038/s41598-019-53997-3
Distribution pattern and molecular signature of cholinergic tuft cells in human gastro-intestinal and pancreatic-biliary tract
Abstract
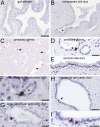
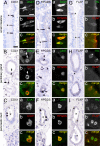
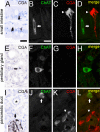
Despite considerable recent insight into the molecular phenotypes and type 2 innate immune functions of tuft cells in rodents, there is sparse knowledge about the region-specific presence and molecular phenotypes of tuft cells in the human digestive tract. Here, we traced cholinergic tuft cells throughout the human alimentary tract with immunohistochemistry and deciphered their region-specific distribution and biomolecule coexistence patterns. While absent from the human stomach, cholinergic tuft cells localized to villi and crypts in the small and large intestines. In the biliary tract, they were present in the epithelium of extra-hepatic peribiliary glands, but not observed in the epithelia of the gall bladder and the common duct of the biliary tract. In the pancreas, solitary cholinergic tuft cells were frequently observed in the epithelia of small and medium-size intra- and inter-lobular ducts, while they were absent from acinar cells and from the main pancreatic duct. Double immunofluorescence revealed co-expression of choline acetyltransferase with structural (cytokeratin 18, villin, advillin) tuft cell markers and eicosanoid signaling (cyclooxygenase 1, hematopoietic prostaglandin D synthase, 5-lipoxygenase activating protein) biomolecules. Our results indicate that region-specific cholinergic signaling of tuft cells plays a role in mucosal immunity in health and disease, especially in infection and cancer.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Advillin is a tuft cell marker in the mouse alimentary tract.J Mol Histol. 2020 Aug;51(4):421-435. doi: 10.1007/s10735-020-09893-6. Epub 2020 Jul 2. J Mol Histol. 2020. PMID: 32617896 Free PMC article.
-
Mouse intestinal tuft cells express advillin but not villin.Sci Rep. 2020 Jun 1;10(1):8877. doi: 10.1038/s41598-020-65469-0. Sci Rep. 2020. PMID: 32483224 Free PMC article.
-
The hepatic, biliary, and pancreatic network of stem/progenitor cell niches in humans: A new reference frame for disease and regeneration.Hepatology. 2016 Jul;64(1):277-86. doi: 10.1002/hep.28326. Epub 2015 Dec 24. Hepatology. 2016. PMID: 26524612 Review.
-
Bile acid-sensitive tuft cells regulate biliary neutrophil influx.Sci Immunol. 2022 Mar 4;7(69):eabj1080. doi: 10.1126/sciimmunol.abj1080. Epub 2022 Mar 4. Sci Immunol. 2022. PMID: 35245089 Free PMC article.
-
Tuft Cells: Context- and Tissue-Specific Programming for a Conserved Cell Lineage.Annu Rev Pathol. 2023 Jan 24;18:311-335. doi: 10.1146/annurev-pathol-042320-112212. Epub 2022 Nov 9. Annu Rev Pathol. 2023. PMID: 36351364 Free PMC article. Review.
Cited by
-
Single-cell atlases: shared and tissue-specific cell types across human organs.Nat Rev Genet. 2022 Jul;23(7):395-410. doi: 10.1038/s41576-022-00449-w. Epub 2022 Feb 25. Nat Rev Genet. 2022. PMID: 35217821 Review.
-
Tuft cell-derived acetylcholine promotes epithelial chloride secretion and intestinal helminth clearance.Immunity. 2024 Jun 11;57(6):1243-1259.e8. doi: 10.1016/j.immuni.2024.03.023. Epub 2024 May 13. Immunity. 2024. PMID: 38744291
-
Cholinergic Mechanisms in Gastrointestinal Neoplasia.Int J Mol Sci. 2024 May 13;25(10):5316. doi: 10.3390/ijms25105316. Int J Mol Sci. 2024. PMID: 38791353 Free PMC article. Review.
-
Helminth Sensing at the Intestinal Epithelial Barrier-A Taste of Things to Come.Front Immunol. 2020 Jul 30;11:1489. doi: 10.3389/fimmu.2020.01489. eCollection 2020. Front Immunol. 2020. PMID: 32849506 Free PMC article. Review.
-
Stem cells and origins of cancer in the upper gastrointestinal tract.Cell Stem Cell. 2021 Aug 5;28(8):1343-1361. doi: 10.1016/j.stem.2021.05.012. Epub 2021 Jun 14. Cell Stem Cell. 2021. PMID: 34129814 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

