Rapid de Novo Preparation of 2,6-Dideoxy Sugar Libraries through Gold-Catalyzed Homopropargyl Orthoester Cyclization
- PMID: 31755271
- PMCID: PMC6956608
- DOI: 10.1021/acs.orglett.9b03812
Rapid de Novo Preparation of 2,6-Dideoxy Sugar Libraries through Gold-Catalyzed Homopropargyl Orthoester Cyclization
Abstract
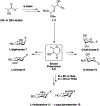
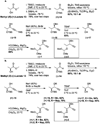
A flexible de novo route capable of producing libraries of 2,6-dideoxy sugars is described. We have found that Au(JackiePhos)SbF6MeCN promotes the conversion of homopropargyl orthoesters into functionalized 2,3-dihydro-4H-pyran-4-ones in good to excellent yields (71-90%). These latter compounds can be easily converted into a number of otherwise difficult to access 2,6-dideoxy sugars.
Figures
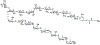
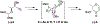
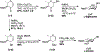
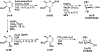
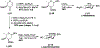
Similar articles
-
Gold-catalyzed sequential alkyne activation for the synthesis of 4,6-disubstituted phosphorus 2-pyrones.Org Lett. 2013 Jan 4;15(1):26-9. doi: 10.1021/ol3029274. Epub 2012 Dec 13. Org Lett. 2013. PMID: 23236965
-
Gold(I)-catalyzed furan-yne cyclizations involving 1,2-rearrangement: efficient synthesis of functionalized 1-naphthols and its application to the synthesis of wailupemycin G.Chemistry. 2014 Sep 15;20(38):12015-9. doi: 10.1002/chem.201403113. Epub 2014 Jul 30. Chemistry. 2014. PMID: 25080016
-
Syntheses of α-pyrones using gold-catalyzed coupling reactions.Org Lett. 2011 Jun 3;13(11):2834-6. doi: 10.1021/ol200794w. Epub 2011 May 2. Org Lett. 2011. PMID: 21534543 Free PMC article.
-
Ligand effects in homogeneous Au catalysis.Chem Rev. 2008 Aug;108(8):3351-78. doi: 10.1021/cr068430g. Epub 2008 Jul 25. Chem Rev. 2008. PMID: 18652511 Free PMC article. Review. No abstract available.
-
Recent Advances in Gold(I)-Catalyzed Approaches to Three-Type Small-Molecule Scaffolds via Arylalkyne Activation.Molecules. 2022 Dec 15;27(24):8956. doi: 10.3390/molecules27248956. Molecules. 2022. PMID: 36558089 Free PMC article. Review.
Cited by
-
Synthesis of C3-epi-virenose and anomerically activated derivatives.Tetrahedron Lett. 2024 Apr;140:155041. doi: 10.1016/j.tetlet.2024.155041. Tetrahedron Lett. 2024. PMID: 38665383 Free PMC article.
-
Automated, Multistep Continuous-Flow Synthesis of 2,6-Dideoxy and 3-Amino-2,3,6-trideoxy Monosaccharide Building Blocks.Angew Chem Int Ed Engl. 2021 Oct 18;60(43):23171-23175. doi: 10.1002/anie.202109887. Epub 2021 Sep 21. Angew Chem Int Ed Engl. 2021. PMID: 34463017 Free PMC article.
-
De Novo Synthesis of an l-Lemonose Thioglycoside Donor from d-Threonine.Tetrahedron Lett. 2021 Jun 8;73:153097. doi: 10.1016/j.tetlet.2021.153097. Epub 2021 Apr 22. Tetrahedron Lett. 2021. PMID: 34176980 Free PMC article.
-
Modular continuous flow synthesis of orthogonally protected 6-deoxy glucose glycals.Org Biomol Chem. 2020 May 6;18(17):3254-3257. doi: 10.1039/d0ob00522c. Org Biomol Chem. 2020. PMID: 32293636 Free PMC article.
References
-
- Kong F; Zhao N; Siegel MM; Janota K; Ashcroft JS; Koehn FE; Borders DB; Carter GT Saccharomicins, Novel Heptadecaglycoside Antibiotics Effective against Multidrug-Resistant Bacteria. J. Am. Chem. Soc 1998, 120, 13301–13311.
-
- Shi SD-H; Hendrickson CL; Marshall AG; Siegel MM; Kong F; Carter GT Structural validation of saccharomicins by high resolution and high mass accuracy fourier transform-ion cyclotron resonance-mass spectrometry and infrared multiphoton dissociation tandem mass spectrometry. J. Am. Soc. Mass Spectrom 1999, 10, 1285–1290.
-
- Williams GJ; Zhang C; Thorson JS Expanding the Promiscuity of a Natural-Product Glycosyltransferase by Directed Evolution. Nat. Chem. Biol 2007, 3, 657–662. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

