Disruption of Oligodendrogenesis Impairs Memory Consolidation in Adult Mice
- PMID: 31753579
- PMCID: PMC7579726
- DOI: 10.1016/j.neuron.2019.10.013
Disruption of Oligodendrogenesis Impairs Memory Consolidation in Adult Mice
Abstract
The generation of myelin-forming oligodendrocytes persists throughout life and is regulated by neural activity. Here we tested whether experience-driven changes in oligodendrogenesis are important for memory consolidation. We found that water maze learning promotes oligodendrogenesis and de novo myelination in the cortex and associated white matter tracts. Preventing these learning-induced increases in oligodendrogenesis without affecting existing oligodendrocytes impaired memory consolidation of water maze, as well as contextual fear, memories. These results suggest that de novo myelination tunes activated circuits, promoting coordinated activity that is important for memory consolidation. Consistent with this, contextual fear learning increased the coupling of hippocampal sharp wave ripples and cortical spindles, and these learning-induced increases in ripple-spindle coupling were blocked when oligodendrogenesis was suppressed. Our results identify a non-neuronal form of plasticity that remodels hippocampal-cortical networks following learning and is required for memory consolidation.
Keywords: Mrf; Oligodendrogenesis; cortex; hippocampus; memory consolidation; myelin.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures
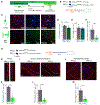
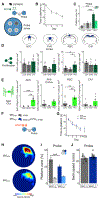

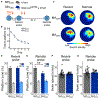

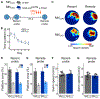

Comment in
-
Wrapping up memories.Nat Rev Neurosci. 2020 Feb;21(2):57. doi: 10.1038/s41583-019-0261-y. Nat Rev Neurosci. 2020. PMID: 31875028 No abstract available.
-
Glia: The Glue Holding Memories Together.Neuron. 2020 Jan 8;105(1):9-11. doi: 10.1016/j.neuron.2019.12.016. Neuron. 2020. PMID: 31951529
-
Myelin makes memories.Nat Neurosci. 2020 Apr;23(4):469-470. doi: 10.1038/s41593-020-0606-x. Nat Neurosci. 2020. PMID: 32094969 Free PMC article.
Similar articles
-
Monosynaptic Hippocampal-Prefrontal Projections Contribute to Spatial Memory Consolidation in Mice.J Neurosci. 2019 Aug 28;39(35):6978-6991. doi: 10.1523/JNEUROSCI.2158-18.2019. Epub 2019 Jul 8. J Neurosci. 2019. PMID: 31285301 Free PMC article.
-
Ripple-triggered stimulation of the locus coeruleus during post-learning sleep disrupts ripple/spindle coupling and impairs memory consolidation.Learn Mem. 2016 Apr 15;23(5):238-48. doi: 10.1101/lm.040923.115. Print 2016 May. Learn Mem. 2016. PMID: 27084931 Free PMC article.
-
Occurrence of Hippocampal Ripples is Associated with Activity Suppression in the Mediodorsal Thalamic Nucleus.J Neurosci. 2019 Jan 16;39(3):434-444. doi: 10.1523/JNEUROSCI.2107-18.2018. Epub 2018 Nov 20. J Neurosci. 2019. PMID: 30459228 Free PMC article.
-
Hippocampal coupling with cortical and subcortical structures in the context of memory consolidation.Neurobiol Learn Mem. 2019 Apr;160:21-31. doi: 10.1016/j.nlm.2018.04.004. Epub 2018 Apr 13. Neurobiol Learn Mem. 2019. PMID: 29660400 Review.
-
Hippocampal ripples as a mode of communication with cortical and subcortical areas.Hippocampus. 2020 Jan;30(1):39-49. doi: 10.1002/hipo.22997. Epub 2018 Nov 13. Hippocampus. 2020. PMID: 30069976 Review.
Cited by
-
Spread of pathological human Tau from neurons to oligodendrocytes and loss of high-firing pyramidal neurons in aging mice.Cell Rep. 2022 Nov 15;41(7):111646. doi: 10.1016/j.celrep.2022.111646. Cell Rep. 2022. PMID: 36384116 Free PMC article.
-
Learning-related contraction of gray matter in rodent sensorimotor cortex is associated with adaptive myelination.Elife. 2022 Nov 9;11:e77432. doi: 10.7554/eLife.77432. Elife. 2022. PMID: 36350292 Free PMC article.
-
Temporal Alterations in White Matter in An App Knock-In Mouse Model of Alzheimer's Disease.eNeuro. 2024 Feb 26;11(2):ENEURO.0496-23.2024. doi: 10.1523/ENEURO.0496-23.2024. Print 2024 Feb. eNeuro. 2024. PMID: 38290851 Free PMC article.
-
Reduced and delayed myelination and volume of corpus callosum in an animal model of Fetal Alcohol Spectrum Disorders partially benefit from voluntary exercise.Sci Rep. 2022 Jun 23;12(1):10653. doi: 10.1038/s41598-022-14752-3. Sci Rep. 2022. PMID: 35739222 Free PMC article.
-
Insufficient Oligodendrocyte Turnover in Optic Nerve Contributes to Age-Related Axon Loss and Visual Deficits.J Neurosci. 2023 Mar 15;43(11):1859-1870. doi: 10.1523/JNEUROSCI.2130-22.2023. Epub 2023 Feb 1. J Neurosci. 2023. PMID: 36725322 Free PMC article.
References
-
- Barres BA, and Raff MC (1993). Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature 361, 258–260. - PubMed
-
- Boyce R, Glasgow SD, Williams S, and Adamantidis A (2016). Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science 352, 812–816. - PubMed
-
- Buzsáki G (1996). The hippocampo-neocortical dialogue. Cereb. Cortex 6, 81–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

