Inhibition of SARS-CoV 3CL protease by flavonoids
- PMID: 31724441
- PMCID: PMC6882434
- DOI: 10.1080/14756366.2019.1690480
Inhibition of SARS-CoV 3CL protease by flavonoids
Abstract
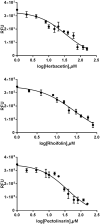
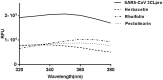
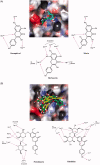
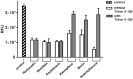
There were severe panics caused by Severe Acute Respiratory Syndrome (SARS) and Middle-East Respiratory Syndrome-Coronavirus. Therefore, researches targeting these viruses have been required. Coronaviruses (CoVs) have been rising targets of some flavonoids. The antiviral activity of some flavonoids against CoVs is presumed directly caused by inhibiting 3C-like protease (3CLpro). Here, we applied a flavonoid library to systematically probe inhibitory compounds against SARS-CoV 3CLpro. Herbacetin, rhoifolin and pectolinarin were found to efficiently block the enzymatic activity of SARS-CoV 3CLpro. The interaction of the three flavonoids was confirmed using a tryptophan-based fluorescence method, too. An induced-fit docking analysis indicated that S1, S2 and S3' sites are involved in binding with flavonoids. The comparison with previous studies showed that Triton X-100 played a critical role in objecting false positive or overestimated inhibitory activity of flavonoids. With the systematic analysis, the three flavonoids are suggested to be templates to design functionally improved inhibitors.
Keywords: FRET; SARS-CoV; SARS-CoV 3CLpro; flavonoid; inhibitory compounds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Flavonoids with inhibitory activity against SARS-CoV-2 3CLpro.J Enzyme Inhib Med Chem. 2020 Dec;35(1):1539-1544. doi: 10.1080/14756366.2020.1801672. J Enzyme Inhib Med Chem. 2020. PMID: 32746637 Free PMC article.
-
Characteristics of flavonoids as potent MERS-CoV 3C-like protease inhibitors.Chem Biol Drug Des. 2019 Dec;94(6):2023-2030. doi: 10.1111/cbdd.13604. Epub 2019 Sep 12. Chem Biol Drug Des. 2019. PMID: 31436895 Free PMC article.
-
The SARS-coronavirus papain-like protease: structure, function and inhibition by designed antiviral compounds.Antiviral Res. 2015 Mar;115:21-38. doi: 10.1016/j.antiviral.2014.12.015. Epub 2014 Dec 29. Antiviral Res. 2015. PMID: 25554382 Free PMC article. Review.
-
Development of a red-shifted fluorescence-based assay for SARS-coronavirus 3CL protease: identification of a novel class of anti-SARS agents from the tropical marine sponge Axinella corrugata.Biol Chem. 2006 Aug;387(8):1063-74. doi: 10.1515/BC.2006.131. Biol Chem. 2006. PMID: 16895476
-
Design and Evaluation of Anti-SARS-Coronavirus Agents Based on Molecular Interactions with the Viral Protease.Molecules. 2020 Aug 27;25(17):3920. doi: 10.3390/molecules25173920. Molecules. 2020. PMID: 32867349 Free PMC article. Review.
Cited by
-
Amentoflavone from Selaginella tamariscina inhibits SARS-CoV-2 RNA-dependent RNA polymerase.Heliyon. 2024 Aug 20;10(16):e36568. doi: 10.1016/j.heliyon.2024.e36568. eCollection 2024 Aug 30. Heliyon. 2024. PMID: 39258186 Free PMC article.
-
Insilico assessment of hesperidin on SARS-CoV-2 main protease and RNA polymerase: Molecular docking and dynamics simulation approach.Biochem Biophys Rep. 2024 Aug 5;39:101804. doi: 10.1016/j.bbrep.2024.101804. eCollection 2024 Sep. Biochem Biophys Rep. 2024. PMID: 39193225 Free PMC article.
-
COVID-19-Associated Sepsis: Potential Role of Phytochemicals as Functional Foods and Nutraceuticals.Int J Mol Sci. 2024 Aug 3;25(15):8481. doi: 10.3390/ijms25158481. Int J Mol Sci. 2024. PMID: 39126050 Free PMC article. Review.
-
Preclinical and Clinical Investigations of Potential Drugs and Vaccines for COVID-19 Therapy: A Comprehensive Review With Recent Update.Clin Pathol. 2024 Jul 26;17:2632010X241263054. doi: 10.1177/2632010X241263054. eCollection 2024 Jan-Dec. Clin Pathol. 2024. PMID: 39070952 Free PMC article. Review.
-
In Vitro Antiviral Activity of Kalanchoe daigremontiana Extract against Human Herpesvirus Type 1.Int J Mol Sci. 2024 Jul 9;25(14):7507. doi: 10.3390/ijms25147507. Int J Mol Sci. 2024. PMID: 39062750 Free PMC article.
References
-
- Drosten C, Günther S, Preiser W, et al. . Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 2003;348:1967–76. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
