Carotenoids as Novel Therapeutic Molecules Against Neurodegenerative Disorders: Chemistry and Molecular Docking Analysis
- PMID: 31703296
- PMCID: PMC6888440
- DOI: 10.3390/ijms20225553
Carotenoids as Novel Therapeutic Molecules Against Neurodegenerative Disorders: Chemistry and Molecular Docking Analysis
Abstract
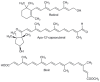
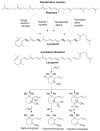
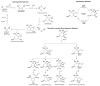
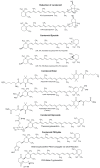
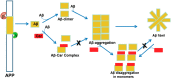
Alzheimer's disease (AD) is the most devastating neurodegenerative disorder that affects the aging population worldwide. Endogenous and exogenous factors are involved in triggering this complex and multifactorial disease, whose hallmark is Amyloid-β (Aβ), formed by cleavage of amyloid precursor protein by β- and γ-secretase. While there is no definitive cure for AD to date, many neuroprotective natural products, such as polyphenol and carotenoid compounds, have shown promising preventive activity, as well as helping in slowing down disease progression. In this article, we focus on the chemistry as well as structure of carotenoid compounds and their neuroprotective activity against Aβ aggregation using molecular docking analysis. In addition to examining the most prevalent anti-amyloidogenic carotenoid lutein, we studied cryptocapsin, astaxanthin, fucoxanthin, and the apocarotenoid bixin. Our computational structure-based drug design analysis and molecular docking simulation revealed important interactions between carotenoids and Aβ via hydrogen bonding and van der Waals interactions, and shows that carotenoids are powerful anti-amyloidogenic molecules with a potential role in preventing AD, especially since most of them can cross the blood-brain barrier and are considered nutraceutical compounds. Our studies thus illuminate mechanistic insights on how carotenoids inhibit Aβ aggregation. The potential role of carotenoids as novel therapeutic molecules in treating AD and other neurodegenerative disorders are discussed.
Keywords: Alzheimer’s disease; Amyloid-β aggregation; apocarotenoid; biosynthesis; carotenoid; molecular docking analysis; structure-activity relationship.
Conflict of interest statement
The authors declare no conflict of interest
Figures





Similar articles
-
Anti-amyloid aggregation activity of novel carotenoids: implications for Alzheimer's drug discovery.Clin Interv Aging. 2017 May 15;12:815-822. doi: 10.2147/CIA.S134605. eCollection 2017. Clin Interv Aging. 2017. PMID: 28553090 Free PMC article.
-
Multiple roles of fucoxanthin and astaxanthin against Alzheimer's disease: Their pharmacological potential and therapeutic insights.Brain Res Bull. 2023 Feb;193:11-21. doi: 10.1016/j.brainresbull.2022.11.018. Epub 2022 Nov 23. Brain Res Bull. 2023. PMID: 36435362 Review.
-
Natural Carotenoids as Neuroprotective Agents for Alzheimer's Disease: An Evidence-Based Comprehensive Review.J Agric Food Chem. 2022 Dec 21;70(50):15631-15646. doi: 10.1021/acs.jafc.2c06206. Epub 2022 Dec 8. J Agric Food Chem. 2022. PMID: 36480951 Review.
-
Biaryl scaffold-focused virtual screening for anti-aggregatory and neuroprotective effects in Alzheimer's disease.BMC Neurosci. 2018 Nov 13;19(1):74. doi: 10.1186/s12868-018-0472-6. BMC Neurosci. 2018. PMID: 30424732 Free PMC article.
-
Inhibition of Alzheimer's amyloid-beta aggregation in-vitro by carbenoxolone: Insight into mechanism of action.Neurochem Int. 2017 Sep;108:481-493. doi: 10.1016/j.neuint.2017.06.011. Epub 2017 Jun 24. Neurochem Int. 2017. PMID: 28652220
Cited by
-
Effects and Mechanisms of Lutein on Aging and Age-Related Diseases.Antioxidants (Basel). 2024 Sep 14;13(9):1114. doi: 10.3390/antiox13091114. Antioxidants (Basel). 2024. PMID: 39334773 Free PMC article. Review.
-
Anti-Inflammatory and Antioxidant Properties of a New Mixture of Vitamin C, Collagen Peptides, Resveratrol, and Astaxanthin in Tenocytes: Molecular Basis for Future Applications in Tendinopathies.Mediators Inflamm. 2024 Jul 30;2024:5273198. doi: 10.1155/2024/5273198. eCollection 2024. Mediators Inflamm. 2024. PMID: 39108992 Free PMC article.
-
Polyphenols with Anti-Inflammatory Properties: Synthesis and Biological Activity of Novel Curcumin Derivatives.Int J Mol Sci. 2023 Feb 12;24(4):3691. doi: 10.3390/ijms24043691. Int J Mol Sci. 2023. PMID: 36835104 Free PMC article.
-
Low Xanthophylls, Retinol, Lycopene, and Tocopherols in Grey and White Matter of Brains with Alzheimer's Disease.J Alzheimers Dis. 2023;94(1):1-17. doi: 10.3233/JAD-220460. J Alzheimers Dis. 2023. PMID: 35988225 Free PMC article. Review.
-
Study on the Synthesis, Antioxidant Properties, and Self-Assembly of Carotenoid-Flavonoid Conjugates.Molecules. 2020 Feb 1;25(3):636. doi: 10.3390/molecules25030636. Molecules. 2020. PMID: 32024181 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

