Human Cytomegalovirus Alters Host Cell Mitochondrial Function during Acute Infection
- PMID: 31694945
- PMCID: PMC6955246
- DOI: 10.1128/JVI.01183-19
Human Cytomegalovirus Alters Host Cell Mitochondrial Function during Acute Infection
Abstract
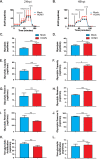
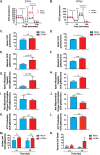
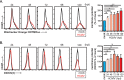
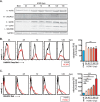
Human cytomegalovirus (HCMV) is a large DNA herpesvirus that is highly prevalent in the human population. HCMV can result in severe direct and indirect pathologies under immunosuppressed conditions and is the leading cause of birth defects related to infectious disease. Currently, the effect of HCMV infection on host cell metabolism as an increase in glycolysis during infection has been defined. We have observed that oxidative phosphorylation is also increased. We have identified morphological and functional changes to host mitochondria during HCMV infection. The mitochondrial network undergoes fission events after HCMV infection. Interestingly, the network does not undergo fusion. At the same time, mitochondrial mass and membrane potential increase. The electron transport chain (ETC) functions at an elevated rate, resulting in the release of increased reactive oxygen species. Surprisingly, despite the stress applied to the host mitochondria, the network is capable of responding to and meeting the increased bioenergetic and biosynthetic demands placed on it. When mitochondrial DNA is depleted from the cells, we observed severe impairment of viral replication. Mitochondrial DNA encodes many of the ETC components. These findings suggest that the host cell ETC is essential to HCMV replication. Our studies suggest the host cell mitochondria may be a therapeutic target.IMPORTANCE Human cytomegalovirus (HCMV) is a herpesvirus present in up to 85% of some populations. Like all herpesviruses, HCMV infection is for life. No vaccine is currently available, neutralizing antibody therapies are ineffective, and current antivirals have limited long-term efficacy due to side effects and potential for viral mutation and resistance. The significance of this research is in understanding how HCMV manipulates the host mitochondria to support bioenergetic and biosynthetic requirements for replication. Despite a large genome, HCMV relies exclusively on host cells for metabolic functions. By understanding the dependency of HCMV on the mitochondria, we could exploit these requirements and develop novel antivirals.
Keywords: CMV; OXPHOS; cytomegalovirus; electron transport chain; membrane potential; mitochondria; mitochondrial biogenesis; mtDNA; oxidative stress; reactive oxygen species.
Copyright © 2020 American Society for Microbiology.
Figures

Similar articles
-
Targeting the Host Mitochondria as a Novel Human Cytomegalovirus Antiviral Strategy.Viruses. 2023 Apr 28;15(5):1083. doi: 10.3390/v15051083. Viruses. 2023. PMID: 37243170 Free PMC article. Review.
-
Human Cytomegalovirus Infection Upregulates the Mitochondrial Transcription and Translation Machineries.mBio. 2016 Mar 29;7(2):e00029. doi: 10.1128/mBio.00029-16. mBio. 2016. PMID: 27025248 Free PMC article.
-
Host Mitochondrial Requirements of Cytomegalovirus Replication.Curr Clin Microbiol Rep. 2020 Dec;7(4):115-123. doi: 10.1007/s40588-020-00153-5. Epub 2020 Sep 30. Curr Clin Microbiol Rep. 2020. PMID: 33816061 Free PMC article.
-
Human Cytomegalovirus pUL37x1 Is Important for Remodeling of Host Lipid Metabolism.J Virol. 2019 Oct 15;93(21):e00843-19. doi: 10.1128/JVI.00843-19. Print 2019 Nov 1. J Virol. 2019. PMID: 31391267 Free PMC article.
-
Systems Virology and Human Cytomegalovirus: Using High Throughput Approaches to Identify Novel Host-Virus Interactions During Lytic Infection.Front Cell Infect Microbiol. 2020 Jun 10;10:280. doi: 10.3389/fcimb.2020.00280. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32587832 Free PMC article. Review.
Cited by
-
Nitric Oxide Circumvents Virus-Mediated Metabolic Regulation during Human Cytomegalovirus Infection.mBio. 2020 Dec 15;11(6):e02630-20. doi: 10.1128/mBio.02630-20. mBio. 2020. PMID: 33323506 Free PMC article.
-
Inhibiting cytomegalovirus replication through targeting the host electron transport chain.Antiviral Res. 2021 Oct;194:105159. doi: 10.1016/j.antiviral.2021.105159. Epub 2021 Aug 11. Antiviral Res. 2021. PMID: 34390771 Free PMC article.
-
Targeting the Host Mitochondria as a Novel Human Cytomegalovirus Antiviral Strategy.Viruses. 2023 Apr 28;15(5):1083. doi: 10.3390/v15051083. Viruses. 2023. PMID: 37243170 Free PMC article. Review.
-
Progression of herpesvirus infection remodels mitochondrial organization and metabolism.PLoS Pathog. 2024 Apr 15;20(4):e1011829. doi: 10.1371/journal.ppat.1011829. eCollection 2024 Apr. PLoS Pathog. 2024. PMID: 38620036 Free PMC article.
-
Development of a CRISPR-Cas12a rapid diagnostic for human cytomegalovirus.Antiviral Res. 2023 Jul;215:105624. doi: 10.1016/j.antiviral.2023.105624. Epub 2023 May 5. Antiviral Res. 2023. PMID: 37150408 Free PMC article.
References
-
- Azevedo LS, Pierrotti LC, Abdala E, Costa SF, Strabelli TMV, Campos SV, Ramos JF, Latif AZA, Litvinov N, Maluf NZ, Caiaffa Filho HH, Pannuti CS, Lopes MH, Santos V. A d, Linardi C. d C G, Yasuda MAS, Marques H. H d S. 2015. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo) 70:515–523. doi:10.6061/clinics/2015(07)09. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

