Proximal Tubule-Derived Amphiregulin Amplifies and Integrates Profibrotic EGF Receptor Signals in Kidney Fibrosis
- PMID: 31676723
- PMCID: PMC6900800
- DOI: 10.1681/ASN.2019030321
Proximal Tubule-Derived Amphiregulin Amplifies and Integrates Profibrotic EGF Receptor Signals in Kidney Fibrosis
Abstract
Background: Sustained activation of EGF receptor (EGFR) in proximal tubule cells is a hallmark of progressive kidney fibrosis after AKI and in CKD. However, the molecular mechanisms and particular EGFR ligands involved are unknown.
Methods: We studied EGFR activation in proximal tubule cells and primary tubular cells isolated from injured kidneys in vitro. To determine in vivo the role of amphiregulin, a low-affinity EGFR ligand that is highly upregulated with injury, we used ischemia-reperfusion injury or unilateral ureteral obstruction in mice with proximal tubule cell-specific knockout of amphiregulin. We also injected soluble amphiregulin into knockout mice with proximal tubule cell-specific deletion of amphiregulin's releasing enzyme, the transmembrane cell-surface metalloprotease, a disintegrin and metalloprotease-17 (ADAM17), and into ADAM17 hypomorphic mice.
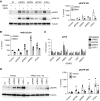
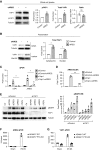
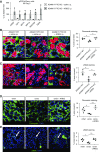
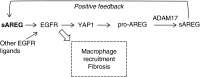
Results: Yes-associated protein 1 (YAP1)-dependent upregulation of amphiregulin transcript and protein amplifies amphiregulin signaling in a positive feedback loop. YAP1 also integrates signals of other moderately injury-upregulated, low-affinity EGFR ligands (epiregulin, epigen, TGFα), which also require soluble amphiregulin and YAP1 to induce sustained EGFR activation in proximal tubule cells in vitro. In vivo, soluble amphiregulin injection sufficed to reverse protection from fibrosis after ischemia-reperfusion injury in ADAM17 hypomorphic mice; injected soluble amphiregulin also reversed the corresponding protective proximal tubule cell phenotype in injured proximal tubule cell-specific ADAM17 knockout mice. Moreover, the finding that proximal tubule cell-specific amphiregulin knockout mice were protected from fibrosis after ischemia-reperfusion injury or unilateral ureteral obstruction demonstrates that amphiregulin was necessary for the development of fibrosis.
Conclusions: Our results identify amphiregulin as a key player in injury-induced kidney fibrosis and suggest therapeutic or diagnostic applications of soluble amphiregulin in kidney disease.
Keywords: chronic kidney disease; epidermal growth factor; fibrosis; proximal tubule.
Copyright © 2019 by the American Society of Nephrology.
Figures




Similar articles
-
ADAM17 substrate release in proximal tubule drives kidney fibrosis.JCI Insight. 2016 Aug 18;1(13):e87023. doi: 10.1172/jci.insight.87023. JCI Insight. 2016. PMID: 27642633 Free PMC article.
-
TNF or EGFR inhibition equally block AKI-to-CKD transition: opportunities for etanercept treatment.Nephrol Dial Transplant. 2023 May 4;38(5):1139-1150. doi: 10.1093/ndt/gfac290. Nephrol Dial Transplant. 2023. PMID: 36269313 Free PMC article.
-
ErbB4 deletion accelerates renal fibrosis following renal injury.Am J Physiol Renal Physiol. 2018 May 1;314(5):F773-F787. doi: 10.1152/ajprenal.00260.2017. Epub 2017 Jul 19. Am J Physiol Renal Physiol. 2018. PMID: 28724608 Free PMC article.
-
Maladaptive proximal tubule repair: cell cycle arrest.Nephron Clin Pract. 2014;127(1-4):61-4. doi: 10.1159/000363673. Epub 2014 Sep 24. Nephron Clin Pract. 2014. PMID: 25343823 Review.
-
TACE/ADAM17 processing of EGFR ligands indicates a role as a physiological convertase.Ann N Y Acad Sci. 2003 May;995:22-38. doi: 10.1111/j.1749-6632.2003.tb03207.x. Ann N Y Acad Sci. 2003. PMID: 12814936 Review.
Cited by
-
Clinical significance of amphiregulin in patients with chronic kidney disease.Clin Exp Nephrol. 2024 May;28(5):421-430. doi: 10.1007/s10157-023-02445-8. Epub 2024 Feb 25. Clin Exp Nephrol. 2024. PMID: 38402497
-
EGFR-mediated activation of adipose tissue macrophages promotes obesity and insulin resistance.Nat Commun. 2022 Aug 10;13(1):4684. doi: 10.1038/s41467-022-32348-3. Nat Commun. 2022. PMID: 35948530 Free PMC article.
-
Tissue regulatory T cells.Immunology. 2020 Sep;161(1):4-17. doi: 10.1111/imm.13208. Epub 2020 Jun 24. Immunology. 2020. PMID: 32463116 Free PMC article. Review.
-
Targeting the epidermal growth factor receptor (EGFR/ErbB) for the potential treatment of renal pathologies.Front Pharmacol. 2024 Aug 21;15:1394997. doi: 10.3389/fphar.2024.1394997. eCollection 2024. Front Pharmacol. 2024. PMID: 39234105 Free PMC article. Review.
-
The Role of the Epidermal Growth Factor Receptor in Diabetic Kidney Disease.Cells. 2022 Oct 28;11(21):3416. doi: 10.3390/cells11213416. Cells. 2022. PMID: 36359813 Free PMC article. Review.
References
-
- Rewa O, Bagshaw SM: Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol 10: 193–207, 2014 - PubMed
-
- Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, et al. .: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 - PubMed
-
- Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, Rouleau JL, et al. .: Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med 351: 1285–1295, 2004 - PubMed
-
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

