Toward Combined Cell and Gene Therapy for Genodermatoses
- PMID: 31653644
- PMCID: PMC7197428
- DOI: 10.1101/cshperspect.a035667
Toward Combined Cell and Gene Therapy for Genodermatoses
Abstract
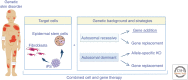
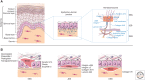
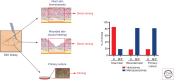
To date, more than 200 monogenic, often devastating, skin diseases have been described. Because of unmet medical needs, development of long-lasting and curative therapies has been consistently attempted, with the aim of correcting the underlying molecular defect. In this review, we will specifically address the few combined cell and gene therapy strategies that made it to the clinics. Based on these studies, what can be envisioned for the future is a patient-oriented strategy, built on the specific features of the individual in need. Most likely, a combination of different strategies, approaches, and advanced therapies will be required to reach the finish line at the end of the long and winding road hampering the achievement of definitive treatments for genodermatoses.
Copyright © 2020 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures

Similar articles
-
Inside out: regenerative medicine for recessive dystrophic epidermolysis bullosa.Pediatr Res. 2018 Jan;83(1-2):318-324. doi: 10.1038/pr.2017.244. Epub 2017 Nov 1. Pediatr Res. 2018. PMID: 29593249 Review.
-
Toward epidermal stem cell-mediated ex vivo gene therapy of junctional epidermolysis bullosa.Hum Gene Ther. 2000 Nov 1;11(16):2283-7. doi: 10.1089/104303400750035825. Hum Gene Ther. 2000. PMID: 11084687 Review.
-
The genodermatoses: candidate diseases for gene therapy.Hum Gene Ther. 2000 Nov 1;11(16):2267-75. doi: 10.1089/104303400750035807. Hum Gene Ther. 2000. PMID: 11084685 Review.
-
Epidermolysis bullosa: where do we stand?Indian J Dermatol Venereol Leprol. 2011 Jul-Aug;77(4):431-8. doi: 10.4103/0378-6323.82393. Indian J Dermatol Venereol Leprol. 2011. PMID: 21727690 Review.
-
Construction of skin equivalents for gene therapy of recessive dystrophic epidermolysis bullosa.Hum Gene Ther. 2004 Oct;15(10):921-33. doi: 10.1089/hum.2004.15.921. Hum Gene Ther. 2004. PMID: 15585108
Cited by
-
A cellular disease model toward gene therapy of TGM1-dependent lamellar ichthyosis.Mol Ther Methods Clin Dev. 2024 Jul 31;32(3):101311. doi: 10.1016/j.omtm.2024.101311. eCollection 2024 Sep 12. Mol Ther Methods Clin Dev. 2024. PMID: 39234443 Free PMC article.
-
Coordinating energy metabolism and signaling pathways in epithelial self-renewal and differentiation.Biol Direct. 2024 Aug 7;19(1):63. doi: 10.1186/s13062-024-00510-0. Biol Direct. 2024. PMID: 39113077 Free PMC article. Review.
-
Current Status of Biomedical Products for Gene and Cell Therapy of Recessive Dystrophic Epidermolysis Bullosa.Int J Mol Sci. 2024 Sep 24;25(19):10270. doi: 10.3390/ijms251910270. Int J Mol Sci. 2024. PMID: 39408598 Free PMC article. Review.
-
Translational perspectives to treat Epidermolysis bullosa-Where do we stand?Exp Dermatol. 2020 Nov;29(11):1112-1122. doi: 10.1111/exd.14194. Exp Dermatol. 2020. PMID: 33043517 Free PMC article.
-
Tracing the Dynamics of Stem Cell Fate.Cold Spring Harb Perspect Biol. 2020 Jun 1;12(6):a036202. doi: 10.1101/cshperspect.a036202. Cold Spring Harb Perspect Biol. 2020. PMID: 31932319 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
