Mitophagy-dependent macrophage reprogramming protects against kidney fibrosis
- PMID: 31639106
- PMCID: PMC6962025
- DOI: 10.1172/jci.insight.132826
Mitophagy-dependent macrophage reprogramming protects against kidney fibrosis
Abstract
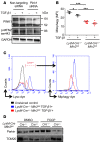
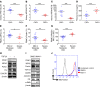
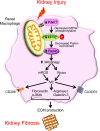
Mitophagy, by maintaining mitochondrial quality control, plays a key role in maintaining kidney function and is impaired in pathologic states. Macrophages are well known for their pathogenic role in kidney fibrosis. Here, we report that PINK1/Parkin-mediated mitophagy in macrophages is compromised in experimental and human kidney fibrosis. We demonstrate downregulation of mitophagy regulators mitofusin-2 (MFN2) and Parkin downstream of PINK1 in kidney fibrosis. Loss of either Pink1 or Prkn promoted renal extracellular matrix accumulation and frequency of profibrotic/M2 macrophages. Pink1-/- or Prkn-/- BM-derived macrophages (BMDMs) showed enhanced expression of rictor. Mitochondria from TGF-β1-treated Pink1-/- BMDMs exhibited increased superoxide levels, along with reduced respiration and ATP production. In addition, mitophagy in macrophages involves PINK1-mediated phosphorylation of downstream MFN2, MFN2-facilitated recruitment of Parkin to damaged mitochondria, and macrophage-specific deletion of Mfn2 aggravates kidney fibrosis. Moreover, mitophagy regulators were downregulated in human CKD kidney and TGF-β1-treated human renal macrophages, whereas Mdivi1 treatment suppressed mitophagy mediators and promoted fibrotic response. Taken together, our study is the first to our knowledge to demonstrate that macrophage mitophagy plays a protective role against kidney fibrosis via regulating the PINK1/MFN2/Parkin-mediated pathway.
Keywords: Fibrosis; Macrophages; Mitochondria; Nephrology.
Conflict of interest statement
Figures


Similar articles
-
Loss of MIEF1/MiD51 confers susceptibility to BAX-mediated cell death and PINK1-PRKN-dependent mitophagy.Autophagy. 2019 Dec;15(12):2107-2125. doi: 10.1080/15548627.2019.1596494. Epub 2019 Mar 28. Autophagy. 2019. PMID: 30894073 Free PMC article.
-
SQSTM1/p62 promotes mitochondrial ubiquitination independently of PINK1 and PRKN/parkin in mitophagy.Autophagy. 2019 Nov;15(11):2012-2018. doi: 10.1080/15548627.2019.1643185. Epub 2019 Jul 24. Autophagy. 2019. PMID: 31339428 Free PMC article.
-
Conditional deletion of myeloid-specific mitofusin 2 but not mitofusin 1 promotes kidney fibrosis.Kidney Int. 2022 May;101(5):963-986. doi: 10.1016/j.kint.2022.01.030. Epub 2022 Feb 26. Kidney Int. 2022. PMID: 35227692 Free PMC article.
-
N-degron-mediated degradation and regulation of mitochondrial PINK1 kinase.Curr Genet. 2020 Aug;66(4):693-701. doi: 10.1007/s00294-020-01062-2. Epub 2020 Mar 10. Curr Genet. 2020. PMID: 32157382 Review.
-
The three 'P's of mitophagy: PARKIN, PINK1, and post-translational modifications.Genes Dev. 2015 May 15;29(10):989-99. doi: 10.1101/gad.262758.115. Genes Dev. 2015. PMID: 25995186 Free PMC article. Review.
Cited by
-
Autophagy in renal fibrosis: Protection or promotion?Front Pharmacol. 2022 Aug 24;13:963920. doi: 10.3389/fphar.2022.963920. eCollection 2022. Front Pharmacol. 2022. PMID: 36105212 Free PMC article. Review.
-
Reprogramming Metabolism of Macrophages as a Target for Kidney Dysfunction Treatment in Autoimmune Diseases.Int J Mol Sci. 2022 Jul 21;23(14):8024. doi: 10.3390/ijms23148024. Int J Mol Sci. 2022. PMID: 35887371 Free PMC article. Review.
-
Loss of O6-methylguanine DNA methyltransferase (MGMT) in macrophages alters responses to TLR3 stimulation and enhances DNA double-strand breaks and mitophagy.Sci Rep. 2024 Nov 11;14(1):27492. doi: 10.1038/s41598-024-78885-3. Sci Rep. 2024. PMID: 39528715 Free PMC article.
-
The Mitochondrion: A Promising Target for Kidney Disease.Pharmaceutics. 2023 Feb 8;15(2):570. doi: 10.3390/pharmaceutics15020570. Pharmaceutics. 2023. PMID: 36839892 Free PMC article. Review.
-
Melatonin Protects Chronic Kidney Disease Mesenchymal Stem/Stromal Cells against Accumulation of Methylglyoxal via Modulation of Hexokinase-2 Expression.Biomol Ther (Seoul). 2022 Jan 1;30(1):28-37. doi: 10.4062/biomolther.2021.058. Biomol Ther (Seoul). 2022. PMID: 34261819 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

