Talin1 knockdown prohibits the proliferation and migration of colorectal cancer cells via the EMT signaling pathway
- PMID: 31612049
- PMCID: PMC6781565
- DOI: 10.3892/ol.2019.10902
Talin1 knockdown prohibits the proliferation and migration of colorectal cancer cells via the EMT signaling pathway
Retraction in
-
Talin1 knockdown prohibits the proliferation and migration of colorectal cancer cells via the EMT signaling pathway.Oncol Lett. 2021 Sep;22(3):682. doi: 10.3892/ol.2021.12943. Epub 2021 Jul 27. Oncol Lett. 2021. PMID: 34434281 Free PMC article.
Abstract
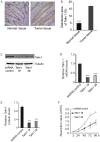
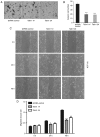
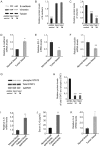
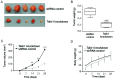
Colorectal cancer (CRC) is the third most commonly diagnosed cancer and the second highest cause of cancer-associated death worldwide. Talin1 activates integrins, which mediate cell adhesion, proliferation, tumorigenesis and metastasis. The aim of the present study was to determine talin1 expression levels in colorectal cancer (CRC) and investigate the role of talin1 in CRC proliferation and invasion in vitro and in vivo. Talin1 protein expression levels were detected in human CRC and adjacent normal tissues by immunohistochemistry. Talin1 short hairpin RNA and control vectors were designed and stably transfected into HCT116 CRC cells. Cell proliferation was determined by MTT assay. Cell migratory and invasive capabilities were detected by wound-healing and Matrigel invasion assays. The expression of proteins in the epithelial-to-mesenchymal transition signaling pathway was determined by western blotting and reverse transcription-quantitative PCR. The effect of talin1 on tumor growth was explored in vivo using BALB/c nude mice. Immunohistochemical analysis of CRC and adjacent normal tissue revealed that talin1 expression was upregulated in CRC. Talin1 knockdown significantly reduced the proliferation, migration and invasive ability of HCT116 cells compared with the control. Protein levels of phosphorylated STAT3 and vimentin were significantly lower in talin1-knockdown HCT116 cell lines compared with the control, whereas protein levels of E-cadherin were increased. Interleukin-6 mRNA levels were significantly increased in patients' blood samples compared with blood samples from healthy controls, as well as in CRC compared with adjacent normal tissue. In vivo experiments demonstrated that talin1 knockdown reduced CRC tumor growth and weight in nude mice. In conclusion, Talin1 knockdown may prevent the proliferation and migration of CRC cells by downregulating various factors involved in the epithelial-to-mesenchymal transition process, such as phosphorylated STAT3 and vimentin; therefore, talin1 may provide a novel therapeutic target for CRC.
Keywords: colorectal cancer; epithelial-to-mesenchymal transition; migration; proliferation; talin1.
Copyright: © Ji et al.
Figures
Similar articles
-
TAGLN2 promotes the proliferation, invasion, migration and epithelial-mesenchymal transition of colorectal cancer cells by activating STAT3 signaling through ANXA2.Oncol Lett. 2021 Oct;22(4):737. doi: 10.3892/ol.2021.12998. Epub 2021 Aug 16. Oncol Lett. 2021. PMID: 34466149 Free PMC article.
-
GRHL2 inhibits colorectal cancer progression and metastasis via oppressing epithelial-mesenchymal transition.Cancer Biol Ther. 2019;20(9):1195-1205. doi: 10.1080/15384047.2019.1599664. Epub 2019 May 7. Cancer Biol Ther. 2019. PMID: 31063022 Free PMC article.
-
STIP1 knockdown suppresses colorectal cancer cell proliferation, migration and invasion by inhibiting STAT3 pathway.Chem Biol Interact. 2021 May 25;341:109446. doi: 10.1016/j.cbi.2021.109446. Epub 2021 Mar 23. Chem Biol Interact. 2021. PMID: 33766539
-
Knockdown of TRIM66 inhibits cell proliferation, migration and invasion in colorectal cancer through JAK2/STAT3 pathway.Life Sci. 2019 Oct 15;235:116799. doi: 10.1016/j.lfs.2019.116799. Epub 2019 Aug 28. Life Sci. 2019. PMID: 31472144
-
MiR-19a-3p regulates the Forkhead box F2-mediated Wnt/β-catenin signaling pathway and affects the biological functions of colorectal cancer cells.World J Gastroenterol. 2020 Feb 14;26(6):627-644. doi: 10.3748/wjg.v26.i6.627. World J Gastroenterol. 2020. PMID: 32103872 Free PMC article.
Cited by
-
Low expression of Talin1 is associated with advanced pathological features in colorectal cancer patients.Sci Rep. 2020 Oct 20;10(1):17786. doi: 10.1038/s41598-020-74810-6. Sci Rep. 2020. PMID: 33082414 Free PMC article.
-
TLN2 functions as a tumor suppressor in clear cell renal cell carcinoma via inactivation of the Wnt/β-catenin signaling pathway.Transl Androl Urol. 2022 Jan;11(1):39-52. doi: 10.21037/tau-21-914. Transl Androl Urol. 2022. PMID: 35242640 Free PMC article.
-
Talin‑1 interaction network in cellular mechanotransduction (Review).Int J Mol Med. 2022 May;49(5):60. doi: 10.3892/ijmm.2022.5116. Epub 2022 Mar 10. Int J Mol Med. 2022. PMID: 35266014 Free PMC article. Review.
-
BCKDK regulates breast cancer cell adhesion and tumor metastasis by inhibiting TRIM21 ubiquitinate talin1.Cell Death Dis. 2023 Jul 17;14(7):445. doi: 10.1038/s41419-023-05944-4. Cell Death Dis. 2023. PMID: 37460470 Free PMC article.
-
High expression of Talin-1 is associated with tumor progression and recurrence in melanoma skin cancer patients.BMC Cancer. 2023 Apr 3;23(1):302. doi: 10.1186/s12885-023-10771-z. BMC Cancer. 2023. PMID: 37013489 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
