A Modified Collagen Dressing Induces Transition of Inflammatory to Reparative Phenotype of Wound Macrophages
- PMID: 31586077
- PMCID: PMC6778115
- DOI: 10.1038/s41598-019-49435-z
A Modified Collagen Dressing Induces Transition of Inflammatory to Reparative Phenotype of Wound Macrophages
Abstract
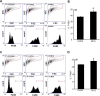
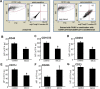
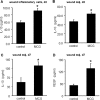
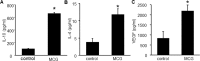
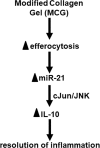
Collagen containing wound-care dressings are extensively used. However, the mechanism of action of these dressings remain unclear. Earlier studies utilizing a modified collagen gel (MCG) dressing demonstrated improved vascularization of ischemic wounds and better healing outcomes. Wound macrophages are pivotal in facilitating wound angiogenesis and timely healing. The current study was designed to investigate the effect of MCG on wound macrophage phenotype and function. MCG augmented recruitment of macrophage at the wound-site, attenuated pro-inflammatory and promoted anti-inflammatory macrophage polarization. Additionally, MCG increased anti-inflammatory IL-10, IL-4 and pro-angiogenic VEGF production, indicating a direct role of MCG in resolving wound inflammation and improving angiogenesis. At the wound-site, impairment in clearance of apoptotic cell bioburden enables chronic inflammation. Engulfment of apoptotic cells by macrophages (efferocytosis) resolves inflammation via a miR-21-PDCD4-IL-10 pathway. MCG-treated wound macrophages exhibited a significantly bolstered efferocytosis index. Such favorable outcome significantly induced miR-21 expression. MCG-mediated IL-10 production was dampened under conditions of miR-21 knockdown pointing towards miR-21 as a causative factor. Pharmacological inhibition of JNK attenuated IL-10 production by MCG, implicating miR-21-JNK pathway in MCG-mediated IL-10 production by macrophages. This work provides direct evidence demonstrating that a collagen-based wound-care dressing may influence wound macrophage function and therefore modify wound inflammation outcomes.
Conflict of interest statement
The dressing and unrestricted research development funding was provided to The Ohio State University by Southwest Technologies. CKS serves as a scientific consultant to Southwest Technologies.
Figures
Similar articles
-
A modified collagen gel dressing promotes angiogenesis in a preclinical swine model of chronic ischemic wounds.Wound Repair Regen. 2014 Nov-Dec;22(6):720-9. doi: 10.1111/wrr.12229. Epub 2015 Jan 8. Wound Repair Regen. 2014. PMID: 25224310 Free PMC article.
-
Engulfment of apoptotic cells by macrophages: a role of microRNA-21 in the resolution of wound inflammation.J Immunol. 2014 Feb 1;192(3):1120-9. doi: 10.4049/jimmunol.1300613. Epub 2014 Jan 3. J Immunol. 2014. PMID: 24391209 Free PMC article.
-
Stabilized collagen matrix dressing improves wound macrophage function and epithelialization.FASEB J. 2019 Feb;33(2):2144-2155. doi: 10.1096/fj.201800352R. Epub 2018 Sep 27. FASEB J. 2019. PMID: 30260708 Free PMC article.
-
Macrophage Differentiation in Normal and Accelerated Wound Healing.Results Probl Cell Differ. 2017;62:353-364. doi: 10.1007/978-3-319-54090-0_14. Results Probl Cell Differ. 2017. PMID: 28455716 Free PMC article. Review.
-
Macrophage Phenotypes in Normal and Diabetic Wound Healing and Therapeutic Interventions.Cells. 2022 Aug 5;11(15):2430. doi: 10.3390/cells11152430. Cells. 2022. PMID: 35954275 Free PMC article. Review.
Cited by
-
Composite Membrane Dressings System with Metallic Nanoparticles as an Antibacterial Factor in Wound Healing.Membranes (Basel). 2022 Feb 13;12(2):215. doi: 10.3390/membranes12020215. Membranes (Basel). 2022. PMID: 35207136 Free PMC article. Review.
-
Oncostatin M Improves Cutaneous Wound Re-Epithelialization and Is Deficient under Diabetic Conditions.J Invest Dermatol. 2022 Mar;142(3 Pt A):679-691.e3. doi: 10.1016/j.jid.2021.04.039. Epub 2021 Sep 15. J Invest Dermatol. 2022. PMID: 34534575 Free PMC article.
-
Enhancing cartilage repair with optimized supramolecular hydrogel-based scaffold and pulsed electromagnetic field.Bioact Mater. 2022 Oct 12;22:312-324. doi: 10.1016/j.bioactmat.2022.10.010. eCollection 2023 Apr. Bioact Mater. 2022. PMID: 36263100 Free PMC article.
-
Roles of MicroRNA-21 in Skin Wound Healing: A Comprehensive Review.Front Pharmacol. 2022 Feb 28;13:828627. doi: 10.3389/fphar.2022.828627. eCollection 2022. Front Pharmacol. 2022. PMID: 35295323 Free PMC article. Review.
-
Efficacy of Topical Application of Chum Salmon (Oncorhynchus keta) Skin-derived Collagen Extracts in Improving Oral Traumatic Ulcer Healing.Contemp Clin Dent. 2024 Apr-Jun;15(2):124-128. doi: 10.4103/ccd.ccd_544_22. Epub 2024 Jul 10. Contemp Clin Dent. 2024. PMID: 39206236 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- DK076566/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)/International
- R01 DK114718/DK/NIDDK NIH HHS/United States
- GM069589/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)/International
- R01 DK076566/DK/NIDDK NIH HHS/United States
- DK114718/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)/International
- GM077185/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)/International
- NR015676/U.S. Department of Health & Human Services | NIH | National Institute of Nursing Research (NINR)/International
- NR013898/U.S. Department of Health & Human Services | NIH | National Institute of Nursing Research (NINR)/International
LinkOut - more resources
Full Text Sources
Research Materials

