Changes in the tumor immune microenvironment in resected recurrent soft tissue sarcomas
- PMID: 31555701
- PMCID: PMC6736819
- DOI: 10.21037/atm.2019.07.43
Changes in the tumor immune microenvironment in resected recurrent soft tissue sarcomas
Abstract
Background: Little is known about how the tumor immune microenvironment (TIME) is modulated in recurrent soft tissue sarcomas (STS).
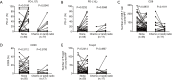
Methods: We evaluated CD8+ T cells, CD20+ B cells, Foxp3+ regulatory T cells (Tregs), and programmed cell death ligand 1 (PD-L1) in 72 paired pre-recurrent (1st resected) versus post-recurrent (2nd resected) STS by immunohistochemistry. Correlations with time to recurrence and prognosis were determined.
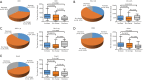
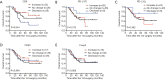
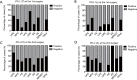
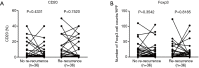
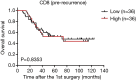
Results: We found that CD8, PD-L1, CD20, and Foxp3-positive cell counts changed in post-recurrent STS. PD-L1-positive tumor cell and lymphocyte counts increased in post-recurrent STS, whereas CD8+ T cell counts decreased. Changes in CD8+ T cell, CD20+ B cell, and PD-L1+ lymphocyte counts were associated with the time interval between surgeries. At admission, fewer CD8+ T cells were detected in patients with relapse than in newly diagnosed patients. Furthermore, post-recurrent STS with fewer CD8+ T cells compared with pre-recurrent STS were more likely to exhibit re-recurrence. The change in CD8+ T cells was positively associated with overall survival. In multivariate analyses, a decrease in CD8+ T cell counts in post-recurrent STS was an independent unfavorable prognostic factor.
Conclusions: The TIME differs between pre-recurrent STS and post-recurrent STS. The variation in CD8+ T cells and PD-L1 positivity may have essential roles during tumor relapse and provides a basis for determining therapeutic strategies.
Keywords: CD8; Soft tissue sarcomas (STS); programmed cell death ligand 1 (PD-L1); recurrence; tumor immune microenvironment (TIME).
Conflict of interest statement
Conflicts of Interest: The authors have no conflicts of interest to declare.
Figures






Similar articles
-
Prognostic impact of the post-treatment T cell composition and spatial organization in soft tissue sarcoma patients treated with neoadjuvant hyperthermic radio(chemo)therapy.Front Immunol. 2023 May 16;14:1185197. doi: 10.3389/fimmu.2023.1185197. eCollection 2023. Front Immunol. 2023. PMID: 37261361 Free PMC article.
-
Tumor-associated macrophages and the tumor immune microenvironment of primary and recurrent epithelial ovarian cancer.Hum Pathol. 2018 Apr;74:135-147. doi: 10.1016/j.humpath.2017.12.010. Epub 2017 Dec 27. Hum Pathol. 2018. PMID: 29288043
-
Stromal PD-L1-Positive Regulatory T cells and PD-1-Positive CD8-Positive T cells Define the Response of Different Subsets of Non-Small Cell Lung Cancer to PD-1/PD-L1 Blockade Immunotherapy.J Thorac Oncol. 2018 Apr;13(4):521-532. doi: 10.1016/j.jtho.2017.11.132. Epub 2017 Dec 18. J Thorac Oncol. 2018. PMID: 29269008
-
PD-L1 Expression Is Associated with FOXP3+ Regulatory T-Cell Infiltration of Soft Tissue Sarcoma and Poor Patient Prognosis.J Cancer. 2017 Jul 5;8(11):2018-2025. doi: 10.7150/jca.18683. eCollection 2017. J Cancer. 2017. PMID: 28819402 Free PMC article.
-
Prognostic Impact of Programmed Death-ligand 1 and Surrounding Immune Status on Stage I Lung Cancer.Clin Lung Cancer. 2020 Jul;21(4):e302-e314. doi: 10.1016/j.cllc.2020.01.013. Epub 2020 Jan 27. Clin Lung Cancer. 2020. PMID: 32102750
Cited by
-
Facts and Hopes in Immunotherapy of Soft-Tissue Sarcomas.Clin Cancer Res. 2020 Nov 15;26(22):5801-5808. doi: 10.1158/1078-0432.CCR-19-3335. Epub 2020 Jun 29. Clin Cancer Res. 2020. PMID: 32601077 Free PMC article. Review.
-
Multi-institutional validation of a radiomics signature for identification of postoperative progression of soft tissue sarcoma.Cancer Imaging. 2024 May 8;24(1):59. doi: 10.1186/s40644-024-00705-8. Cancer Imaging. 2024. PMID: 38720384 Free PMC article.
-
Analysis of tumor-infiltrating NK and T cells highlights IL-15 stimulation and TIGIT blockade as a combination immunotherapy strategy for soft tissue sarcomas.J Immunother Cancer. 2020 Nov;8(2):e001355. doi: 10.1136/jitc-2020-001355. J Immunother Cancer. 2020. PMID: 33158916 Free PMC article.
-
The Immune Contexture of Liposarcoma and Its Clinical Implications.Cancers (Basel). 2022 Sep 21;14(19):4578. doi: 10.3390/cancers14194578. Cancers (Basel). 2022. PMID: 36230502 Free PMC article. Review.
-
Candidate Biomarkers for Specific Intraoperative Near-Infrared Imaging of Soft Tissue Sarcomas: A Systematic Review.Cancers (Basel). 2021 Feb 1;13(3):557. doi: 10.3390/cancers13030557. Cancers (Basel). 2021. PMID: 33535618 Free PMC article. Review.
References
-
- Luen SJ, Salgado R, Fox S, et al. Tumour-infiltrating lymphocytes in advanced HER2-positive breast cancer treated with pertuzumab or placebo in addition to trastuzumab and docetaxel: a retrospective analysis of the CLEOPATRA study. Lancet Oncol 2017;18:52-62. 10.1016/S1470-2045(16)30631-3 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
