Mesenchymal stem cell therapy assisted by nanotechnology: a possible combinational treatment for brain tumor and central nerve regeneration
- PMID: 31534331
- PMCID: PMC6681156
- DOI: 10.2147/IJN.S217923
Mesenchymal stem cell therapy assisted by nanotechnology: a possible combinational treatment for brain tumor and central nerve regeneration
Abstract
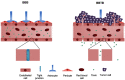
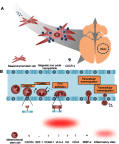
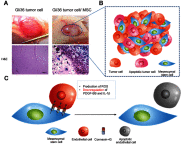
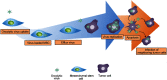
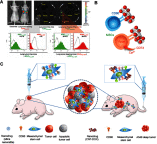
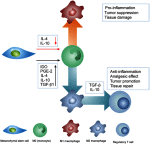
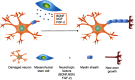
Mesenchymal stem cells (MSCs) intrinsically possess unique features that not only help in their migration towards the tumor-rich environment but they also secrete versatile types of secretomes to induce nerve regeneration and analgesic effects at inflammatory sites. As a matter of course, engineering MSCs to enhance their intrinsic abilities is growing in interest in the oncology and regenerative field. However, the concern of possible tumorigenesis of genetically modified MSCs prompted the development of non-viral transfected MSCs armed with nanotechnology for more effective cancer and regenerative treatment. Despite the fact that a large number of successful studies have expanded our current knowledge in tumor-specific targeting, targeting damaged brain site remains enigmatic due to the presence of a blood-brain barrier (BBB). A BBB is a barrier that separates blood from brain, but MSCs with intrinsic features of transmigration across the BBB can efficiently deliver desired drugs to target sites. Importantly, MSCs, when mediated by nanoparticles, can further enhance tumor tropism and can regenerate the damaged neurons in the central nervous system through the promotion of axon growth. This review highlights the homing and nerve regenerative abilities of MSCs in order to provide a better understanding of potential cell therapeutic applications of non-genetically engineered MSCs with the aid of nanotechnology.
Keywords: anti-inflammation; glioblastoma multiforme; mesenchymal stem cell; nanocarrier; nerve regeneration; tumor inhibition.
Conflict of interest statement
The authors state that there are no conflicts of interest in this work.
Figures

Similar articles
-
Tropism of mesenchymal stem cell toward CD133+ stem cell of glioblastoma in vitro and promote tumor proliferation in vivo.Stem Cell Res Ther. 2018 Nov 9;9(1):310. doi: 10.1186/s13287-018-1049-0. Stem Cell Res Ther. 2018. PMID: 30413179 Free PMC article.
-
Polylysine-modified polyethylenimine polymer can generate genetically engineered mesenchymal stem cells for combinational suicidal gene therapy in glioblastoma.Acta Biomater. 2018 Oct 15;80:144-153. doi: 10.1016/j.actbio.2018.09.015. Epub 2018 Sep 15. Acta Biomater. 2018. PMID: 30223091
-
Nanotechnology and bioengineering approaches to improve the potency of mesenchymal stem cell as an off-the-shelf versatile tumor delivery vehicle.Med Res Rev. 2024 Jul;44(4):1596-1661. doi: 10.1002/med.22023. Epub 2024 Feb 1. Med Res Rev. 2024. PMID: 38299924 Review.
-
Ionizing radiation augments glioma tropism of mesenchymal stem cells.J Neurosurg. 2018 Jan;128(1):287-295. doi: 10.3171/2016.9.JNS16278. Epub 2017 Mar 31. J Neurosurg. 2018. PMID: 28362237 Free PMC article.
-
Biological Aspects and Clinical Applications of Mesenchymal Stem Cells: Key Features You Need to be Aware of.Curr Pharm Biotechnol. 2021;22(2):200-215. doi: 10.2174/1389201021666200907121530. Curr Pharm Biotechnol. 2021. PMID: 32895040 Review.
Cited by
-
Mesenchymal stem cell carriers enhance anti-tumor efficacy of oncolytic virotherapy.Oncol Lett. 2021 Apr;21(4):238. doi: 10.3892/ol.2021.12499. Epub 2021 Jan 28. Oncol Lett. 2021. PMID: 33664802 Free PMC article. Review.
-
Precision Nanomedicine with Bio-Inspired Nanosystems: Recent Trends and Challenges in Mesenchymal Stem Cells Membrane-Coated Bioengineered Nanocarriers in Targeted Nanotherapeutics.J Xenobiot. 2024 Jun 24;14(3):827-872. doi: 10.3390/jox14030047. J Xenobiot. 2024. PMID: 39051343 Free PMC article. Review.
-
Stem Cell Mimicking Nanoencapsulation for Targeting Arthritis.Int J Nanomedicine. 2021 Dec 31;16:8485-8507. doi: 10.2147/IJN.S334298. eCollection 2021. Int J Nanomedicine. 2021. PMID: 35002240 Free PMC article. Review.
-
Smart Nanoformulations for Brain Cancer Theranostics: Challenges and Promises.Cancers (Basel). 2022 Nov 1;14(21):5389. doi: 10.3390/cancers14215389. Cancers (Basel). 2022. PMID: 36358807 Free PMC article. Review.
-
Treating Metastatic Brain Cancers With Stem Cells.Front Mol Neurosci. 2021 Nov 24;14:749716. doi: 10.3389/fnmol.2021.749716. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34899179 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

