A Replication-Defective Human Cytomegalovirus Vaccine Elicits Humoral Immune Responses Analogous to Those with Natural Infection
- PMID: 31511385
- PMCID: PMC6854503
- DOI: 10.1128/JVI.00747-19
A Replication-Defective Human Cytomegalovirus Vaccine Elicits Humoral Immune Responses Analogous to Those with Natural Infection
Abstract
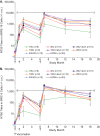
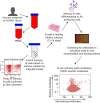
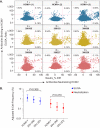
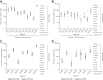
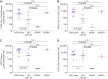
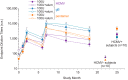
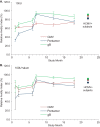
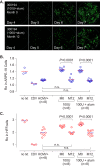
Human cytomegalovirus (HCMV) can cause congenital infections, which are a leading cause of childhood disabilities. Since the rate of maternal-fetal transmission is much lower in naturally infected (HCMV-seropositive) women, we hypothesize that a vaccine candidate capable of eliciting immune responses analogous to those of HCMV-seropositive subjects may confer protection against congenital HCMV. We have previously described a replication-defective virus vaccine based on strain AD169 (D. Wang, D. C. Freed, X. He, F. Li, et al., Sci Transl Med 8:362ra145, 2016, https://doi.org/10.1126/scitranslmed.aaf9387). The vaccine, named V160, has been shown to be safe and immunogenic in HCMV-seronegative human subjects, eliciting both humoral and cellular immune responses (S. P. Adler, S. E. Starr, S. A. Plotkin, S. H. Hempfling, et al., J Infect Dis 220:411-419, 2019, https://doi.org/10.1093/infdis/171.1.26). Here, we further showed that sera from V160-immunized HCMV-seronegative subjects have attributes similar in quality to those from seropositive subjects, including high-avidity antibodies to viral antigens, coverage against a panel of genetically distinct clinical isolates, and protection against viral infection in diverse types of human cells in culture. More importantly, vaccination appeared efficient in priming the human immune system, inducing memory B cells in six V160 recipients at frequencies comparable to those of three HCMV-seropositive subjects. Our results demonstrate the ability of V160 to induce robust and durable humoral memory responses to HCMV, justifying further clinical evaluation of the vaccine against congenital HCMV.IMPORTANCEIn utero HCMV infection can lead to miscarriage or childhood disabilities, and an effective vaccine is urgently needed. Since children born to women who are seropositive prior to pregnancy are less likely to be affected by congenital HCMV infection, it has been hypothesized that a vaccine capable of inducing an immune response resembling the responses in HCMV-seropositive women may be effective. We previously described a replication-defective virus vaccine that has been demonstrated safe and immunogenic in HCMV-seronegative subjects. Here, we conducted additional analyses to show that the vaccine can induce antibodies with functional attributes similar to those from HCMV-seropositive subjects. Importantly, vaccination can induce long-lived memory B cells at frequencies comparable to those seen in HCMV-seropositive subjects. We conclude that this vaccine is a promising candidate that warrants further clinical evaluation for prevention of congenital HCMV.
Keywords: CMV; V160; humoral immunity; memory; neutralization; vaccine.
Copyright © 2019 American Society for Microbiology.
Figures
Similar articles
-
Phase 1 Clinical Trial of a Conditionally Replication-Defective Human Cytomegalovirus (CMV) Vaccine in CMV-Seronegative Subjects.J Infect Dis. 2019 Jul 2;220(3):411-419. doi: 10.1093/infdis/jiz141. J Infect Dis. 2019. PMID: 31535143 Clinical Trial.
-
Neutralization of Diverse Human Cytomegalovirus Strains Conferred by Antibodies Targeting Viral gH/gL/pUL128-131 Pentameric Complex.J Virol. 2017 Mar 13;91(7):e02033-16. doi: 10.1128/JVI.02033-16. Print 2017 Apr 1. J Virol. 2017. PMID: 28077654 Free PMC article.
-
Multiantigenic Modified Vaccinia Virus Ankara Vaccine Vectors To Elicit Potent Humoral and Cellular Immune Reponses against Human Cytomegalovirus in Mice.J Virol. 2018 Sep 12;92(19):e01012-18. doi: 10.1128/JVI.01012-18. Print 2018 Oct 1. J Virol. 2018. PMID: 30045984 Free PMC article.
-
Prospects of a vaccine for the prevention of congenital cytomegalovirus disease.Med Microbiol Immunol. 2016 Dec;205(6):537-547. doi: 10.1007/s00430-016-0472-z. Epub 2016 Aug 12. Med Microbiol Immunol. 2016. PMID: 27519596 Review.
-
Human cytomegalovirus (HCMV) infection/re-infection: development of a protective HCMV vaccine.New Microbiol. 2019 Jan;42(1):1-20. Epub 2019 Jan 21. New Microbiol. 2019. PMID: 30671581 Review.
Cited by
-
Development of broadly neutralizing antibodies targeting the cytomegalovirus subdominant antigen gH.Commun Biol. 2022 Apr 25;5(1):387. doi: 10.1038/s42003-022-03294-z. Commun Biol. 2022. PMID: 35468974 Free PMC article.
-
Pathogenesis of human cytomegalovirus in the immunocompromised host.Nat Rev Microbiol. 2021 Dec;19(12):759-773. doi: 10.1038/s41579-021-00582-z. Epub 2021 Jun 24. Nat Rev Microbiol. 2021. PMID: 34168328 Free PMC article. Review.
-
Clinical characteristics of hospitalized mild/moderate COVID-19 patients with a prolonged negative conversion time of SARS-CoV-2 nucleic acid detection.BMC Infect Dis. 2021 Feb 3;21(1):141. doi: 10.1186/s12879-021-05851-z. BMC Infect Dis. 2021. PMID: 33535989 Free PMC article.
-
Immune Prophylaxis and Therapy for Human Cytomegalovirus Infection.Int J Mol Sci. 2021 Aug 13;22(16):8728. doi: 10.3390/ijms22168728. Int J Mol Sci. 2021. PMID: 34445434 Free PMC article. Review.
-
A conditionally replication-defective cytomegalovirus vaccine elicits potent and diverse functional monoclonal antibodies in a phase I clinical trial.NPJ Vaccines. 2021 Jun 2;6(1):79. doi: 10.1038/s41541-021-00342-3. NPJ Vaccines. 2021. PMID: 34078915 Free PMC article.
References
-
- Mocarski ES, Shenk T, Griffiths PD, Pass RF. 2013. Cytomegaloviruses, p 1960–2014. In Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Rancaniello VR, Roizman B (ed), Fields virology, 6th ed Lippincott Williams & Wilkins, Philadelphia, PA.
-
- Ross SA, Novak Z, Pati S, Patro RK, Blumenthal J, Danthuluri VR, Ahmed A, Michaels MG, Sanchez PJ, Bernstein DI, Tolan RW, Palmer AL, Britt WJ, Fowler KB, Boppana SB. 2011. Mixed infection and strain diversity in congenital cytomegalovirus infection. J Infect Dis 204:1003–1007. doi:10.1093/infdis/jir457. - DOI - PMC - PubMed
-
- Ljungman P, de la Camara R, Cordonnier C, Einsele H, Engelhard D, Reusser P, Styczynski J, Ward K. 2008. Management of CMV, HHV-6, HHV-7 and Kaposi-sarcoma herpesvirus (HHV-8) infections in patients with hematological malignancies and after SCT. Bone Marrow Transplant 42:227–240. doi:10.1038/bmt.2008.162. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

