Regulator of G protein signaling 12 enhances osteoclastogenesis by suppressing Nrf2-dependent antioxidant proteins to promote the generation of reactive oxygen species
- PMID: 31490121
- PMCID: PMC6731062
- DOI: 10.7554/eLife.42951
Regulator of G protein signaling 12 enhances osteoclastogenesis by suppressing Nrf2-dependent antioxidant proteins to promote the generation of reactive oxygen species
Abstract
Regulators of G-protein Signaling are a conserved family of proteins required in various biological processes including cell differentiation. We previously demonstrated that Rgs12 is essential for osteoclast differentiation and its deletion in vivo protected mice against pathological bone loss. To characterize its mechanism in osteoclastogenesis, we selectively deleted Rgs12 in C57BL/6J mice targeting osteoclast precursors using LyzM-driven Cre mice or overexpressed Rgs12 in RAW264.7 cells. Rgs12 deletion in vivo led to an osteopetrotic phenotype evidenced by increased trabecular bone, decreased osteoclast number and activity but no change in osteoblast number and bone formation. Rgs12 overexpression increased osteoclast number and size, and bone resorption activity. Proteomics analysis of Rgs12-depleted osteoclasts identified an upregulation of antioxidant enzymes under the transcriptional regulation of Nrf2, the master regulator of oxidative stress. We confirmed an increase of Nrf2 activity and impaired reactive oxygen species production in Rgs12-deficient cells. Conversely, Rgs12 overexpression suppressed Nrf2 through a mechanism dependent on the 26S proteasome, and promoted RANKL-induced phosphorylation of ERK1/2 and NFκB, which was abrogated by antioxidant treatment. Our study therefore identified a novel role of Rgs12 in regulating Nrf2, thereby controlling cellular redox state and osteoclast differentiation.
Keywords: cell biology; mouse; osteoclasts; proteomics; reactive oxygen species.
© 2019, Ng et al.
Conflict of interest statement
AN, ZL, MJ, SY, CL, CF, CT, MO, JQ, SY No competing interests declared
Figures
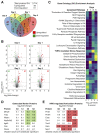
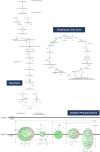
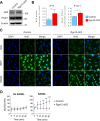


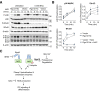

Similar articles
-
Regulators of G protein signaling 12 promotes osteoclastogenesis in bone remodeling and pathological bone loss.Cell Death Differ. 2015 Dec;22(12):2046-57. doi: 10.1038/cdd.2015.45. Epub 2015 Apr 24. Cell Death Differ. 2015. PMID: 25909889 Free PMC article.
-
Deficiency of optineurin enhances osteoclast differentiation by attenuating the NRF2-mediated antioxidant response.Exp Mol Med. 2021 Apr;53(4):667-680. doi: 10.1038/s12276-021-00596-w. Epub 2021 Apr 16. Exp Mol Med. 2021. PMID: 33864025 Free PMC article.
-
Regulator of G Protein Signaling Protein 12 (Rgs12) Controls Mouse Osteoblast Differentiation via Calcium Channel/Oscillation and Gαi-ERK Signaling.J Bone Miner Res. 2019 Apr;34(4):752-764. doi: 10.1002/jbmr.3645. Epub 2019 Jan 28. J Bone Miner Res. 2019. PMID: 30489658 Free PMC article.
-
The Keap1/Nrf2 protein axis plays a role in osteoclast differentiation by regulating intracellular reactive oxygen species signaling.J Biol Chem. 2013 Aug 9;288(32):23009-20. doi: 10.1074/jbc.M113.478545. Epub 2013 Jun 25. J Biol Chem. 2013. PMID: 23801334 Free PMC article.
-
RANKL induces Bach1 nuclear import and attenuates Nrf2-mediated antioxidant enzymes, thereby augmenting intracellular reactive oxygen species signaling and osteoclastogenesis in mice.FASEB J. 2017 Feb;31(2):781-792. doi: 10.1096/fj.201600826R. Epub 2016 Nov 11. FASEB J. 2017. PMID: 27836987
Cited by
-
Puerarin alleviates osteoporosis in the ovariectomy-induced mice by suppressing osteoclastogenesis via inhibition of TRAF6/ROS-dependent MAPK/NF-κB signaling pathways.Aging (Albany NY). 2020 Nov 7;12(21):21706-21729. doi: 10.18632/aging.103976. Epub 2020 Nov 7. Aging (Albany NY). 2020. PMID: 33176281 Free PMC article.
-
Inhibition of insulin degrading enzyme suppresses osteoclast hyperactivity via enhancing Nrf2-dependent antioxidant response in glucocorticoid-induced osteonecrosis of the femoral head.Mol Med. 2024 Jul 31;30(1):111. doi: 10.1186/s10020-024-00880-1. Mol Med. 2024. PMID: 39085816 Free PMC article.
-
RGS12 polarizes the GPSM2-GNAI complex to organize and elongate stereocilia in sensory hair cells.Sci Adv. 2022 Oct 21;8(42):eabq2826. doi: 10.1126/sciadv.abq2826. Epub 2022 Oct 19. Sci Adv. 2022. PMID: 36260679 Free PMC article.
-
TAZ is required for chondrogenesis and skeletal development.Cell Discov. 2021 Apr 20;7(1):26. doi: 10.1038/s41421-021-00254-5. Cell Discov. 2021. PMID: 33879790 Free PMC article.
-
The Roles of ROS Generation in RANKL-Induced Osteoclastogenesis: Suppressive Effects of Febuxostat.Cancers (Basel). 2020 Apr 9;12(4):929. doi: 10.3390/cancers12040929. Cancers (Basel). 2020. PMID: 32283857 Free PMC article.
References
-
- Abiko Y, Miura T, Phuc BH, Shinkai Y, Kumagai Y. Participation of covalent modification of Keap1 in the activation of Nrf2 by tert-butylbenzoquinone, an electrophilic metabolite of butylated hydroxyanisole. Toxicology and Applied Pharmacology. 2011;255:32–39. doi: 10.1016/j.taap.2011.05.013. - DOI - PubMed
-
- Bartell SM, Kim H-N, Ambrogini E, Han L, Iyer S, Serra Ucer S, Rabinovitch P, Jilka RL, Weinstein RS, Zhao H, O’Brien CA, Manolagas SC, Almeida M. FoxO proteins restrain osteoclastogenesis and bone resorption by attenuating H2O2 accumulation. Nature Communications. 2014;5:e3773. doi: 10.1038/ncomms4773. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

